Defining Vaginal Community Dynamics: daily microbiome transitions, the role of menstruation, bacteriophages and bacterial genes
By Assoc Prof Ina Schuppe Koistinen
Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections
About this article
Author
Comments on the article by Hugerth et al. (Microbiome 2024) 1
This high-resolution metagenomic study investigates daily transitions in the vaginal microbiome across a menstrual cycle in 49 healthy young women. By analysing taxonomic, viral, and functional gene data from daily samples, the authors introduce a dynamic classification system called Vaginal Community Dynamics (VCDs), which categorises women into four groups: constant eubiotic, constant dysbiotic, menses-related dysbiosis, and unstable dysbiotic. These patterns reflect how individual microbiomes respond to menstruation, sexual activity, and other exposures, and demonstrate that vaginal health cannot be adequately assessed from static samples alone. Notably, bacteriophage abundance and bacterial gene content — such as bacteriocins — may contribute to the stability or instability of microbial communities. This work highlights the complexity and individuality of vaginal microbiome behaviour and has implications for improving diagnostics and personalised care in gynaecology.
What do we already know about this subject?
The vaginal microbiota plays a key role in defending against pathogens, maintaining mucosal immunity, and supporting reproductive health. Dominance by Lactobacillus species, especially L. crispatus, maintains a low pH and inhibits pathogenic colonisation2 . Dysbiosis — defined by a loss of lactobacilli and overgrowth of anaerobic species such as Gardnerella or Prevotella — is associated with increased risks of bacterial vaginosis (BV), preterm birth3 , infertility4, sexually transmitted infections, human papillomavirus (HPV) infections and gynaecological cancers5 . Previous studies have shown that hormonal changes, menstruation, and sexual intercourse can influence the composition of the vaginal microbiome6. Many of these studies relied on infrequent sampling and lacked resolution to assess short-term fluctuations or determine the drivers of transitions between eubiosis and dysbiosis. The contributions of viral dynamics and functional bacterial genes have remained largely unexplored.
What are the main insights from the study?
This study introduces the concept of VCDs, offering a new framework for classifying microbiome behaviour across the menstrual cycle. Unlike community state types (CSTs), which describe static microbiome compositions, VCDs capture temporal patterns that may better reflect microbiome resilience and vulnerability. The four VCDs — constant eubiotic, constant dysbiotic, menses-related dysbiosis, and unstable dysbiotic — represent distinct profiles of microbial stability. Women in the constant eubiotic group maintained Lactobacillus dominance throughout the cycle, while those with constant dysbiosis had persistent BV-associated communities. Menses-related dysbiosis was characterised by temporary shifts during menstruation, often reverting mid-cycle, whereas the unstable group experienced abrupt fluctuations after exposures like sexual intercourse, suggesting greater ecological fragility.
One of the key findings was that instability in the vaginal microbiome is associated with increased bacteriophage activity and a higher prevalence of L. iners. This species is frequently linked to transitional or less stable states, and phage abundance may reflect active lytic cycles that destabilise dominant bacteria via “kill-the-winner” dynamics. Additionally, women with transient dysbiosis showed increased abundance of potential pathogens such as Sneathia spp. during and after menstruation, pointing to specific periods of vulnerability.
Strain-level analysis revealed differences in bacterial gene content, including bacteriocins produced by Gardnerella leopoldii that may inhibit lactobacilli. These genes were more prevalent in unstable and dysbiotic VCDs, supporting a possible mechanistic role in shaping community structure. Although these genetic findings require further validation, they highlight the importance of moving beyond species-level classification to understand microbial function and its impact on host health.
What are the consequences for clinical practice?
This study underscores the need to rethink how vaginal health is assessed and monitored in clinical practice. The recognition that vaginal microbiota are dynamic — and that stability patterns differ markedly between women — has implications for diagnostics, risk assessment, and therapeutic strategies. Sampling at a single time point, especially during menstruation, may fail to capture meaningful fluctuations or misrepresent a woman’s baseline microbial state. Clinicians should consider collecting samples at multiple points in the cycle to better assess microbiome behavior, particularly in patients with recurrent symptoms or reproductive concerns.
The limitations of CST-based classification are evident in this study. Two women with the same CST may exhibit entirely different VCDs, one with stable eubiosis and the other with frequent dysbiosis. Incorporating VCD assessment could enable more personalised interventions, such as recommending prophylactic microbiome support for women with unstable patterns or targeting high-risk windows (e.g. post-menses) for infection screening.
The identification of phage-driven instability and strain-level bacterial traits opens avenues for precision medicine. Future therapies may need to address microbial function — such as biofilm formation or bacteriocin production — rather than composition alone. Understanding the dynamics of vaginal bacteriophages could also inform novel microbiome stabilisation strategies.
Figure 1. Vaginal time series can be classified into four categories (Vaginal Community Dynamics) according to their proportions of eubiotic samples
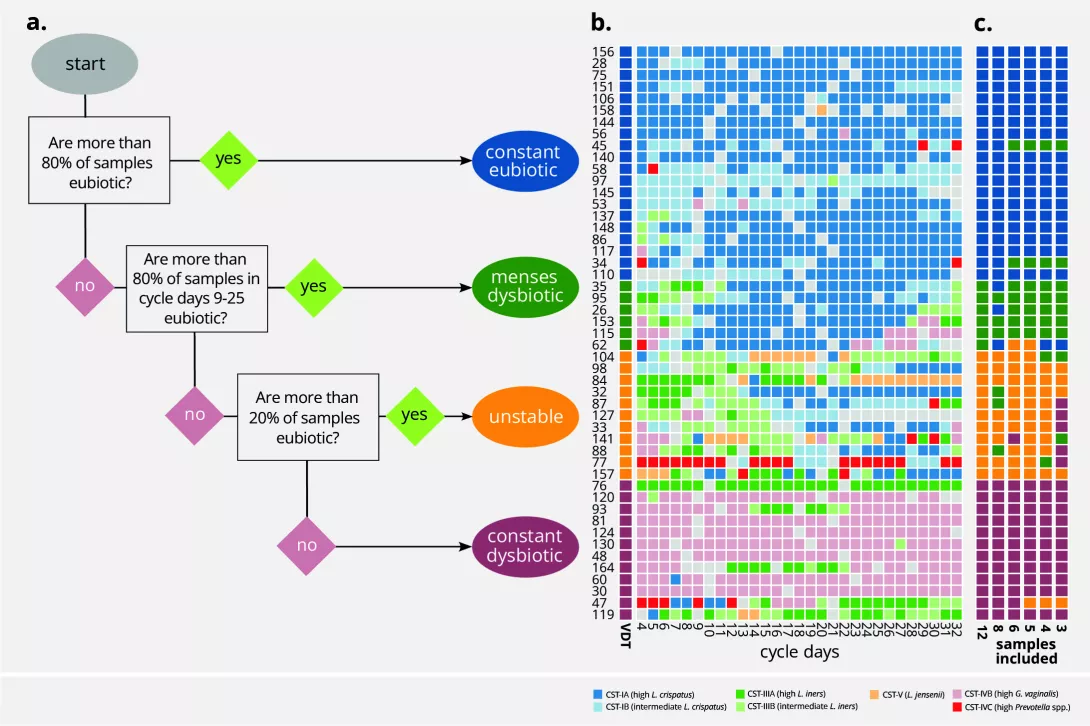
a. A decision tree can separate a time series of samples into dynamic groups, based on the community state types (CSTs). Input from the user determines which CSTs are considered eubiotic (here: I, II, and V) and which days are to be considered free from the influence of menses (here: cycle day 9 to cycle day 25). Time series with ≥80% eubiotic samples are considered constant eubiotic; conversely, those with >80% dysbiotic samples are considered constant dysbiotic. For those in the 20–80% range, a second assessment is done on the days free of menses: if they are >80% eubiotic, the time series is considered menses-related dysbiotic, and otherwise unstable (changing from eubiosis to dysbiosis without a clear temporal pattern). b. A colour map with one individual per row and one day per column. The colour of each intersection depicts CST. Coloured bars on the left side show the vaginal community dynamics of each woman. c. Additional colour bars show the inferred vaginal community dynamics of each participant when using fewer samples for classification. Reproduced from Hugerth LW, et al. Microbiome 2024, 12, 1531 (doi:10.1186/s40168- 024-01870-5) under a CC-BY 4.0 license (creativecommons.org/licenses/by/4.0). No changes have been made to the figure.
KEY POINTS
- The vaginal microbiome shows individual and dynamic patterns during the menstrual cycle that may affect reproductive outcomes.
- Transient or unstable dysbiosis is associated with higher phage counts, Lactobacillus iners dominance, and phases of increased risk.
- Strain-level functional traits, such as bacteriocin production, may help explain transitions to and persistence of dysbiosis.
CONCLUSION
This study presents a significant advance in our understanding of vaginal microbiome behaviour by shifting the focus from static CSTs to dynamic community patterns. By classifying women into four categories of VCDs, the study offers a new lens for evaluating microbiome health and its clinical consequences. These insights call for more personalised, time-sensitive approaches to sampling, diagnosis, and intervention. Incorporating virome data and functional bacterial traits may further refine risk prediction and treatment strategies. Ultimately, a deeper ecological understanding of the vaginal microbiome could help reduce complications like bacterial vaginosis, preterm birth, and infertility — and support a more individualised standard of care for women’s reproductive health.


