This one gut microbe could change the way cancer therapy performs
A single gut microbe may influence whether a patient responds to cancer immunotherapy. This study reveals how signals from the intestine can strengthen the body’s antitumour defences in ways we never expected.
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
About this article
Immunotherapy is rewriting oncology, but in most solid tumours, (sidenote: PD-1 inhibitors Drugs that release the “brakes” on T cells by blocking the PD-1 receptor, allowing the immune system to attack tumours more effectively. ) still fail the majority of patients. Our story starts in a place every clinician knows matters but few can yet “dose”: the gut microbiome. Oncologists have seen that some patients ride a wave of durable response to treatment, while others, seemingly similar on paper, barely respond at all. A new study published in Nature 1 asks a simple but profound question: a single gut microbe that can reprogram dendritic cells, send them on a “road trip” from the intestine to the tumour, and make checkpoint inhibitors work better? And the answer appears to be yes.
A hidden player in PD-1 response
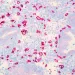
Researchers from National Cancer Center Research Institute, Tokyo followed patients with lung and gastric cancer receiving PD-1 blockade and looked at their stool microbiome just before treatment. Responders consistently had richer bacterial diversity and, more specifically, more members of the
(sidenote:
Ruminococcaceae
A family of gut bacteria often linked to a healthier, more diverse microbiome and better immune activation.
)
family. From this group they isolated an unassuming anaerobe, a strain of Hominenteromicrobium mulieris they call YB328.
Patients with higher levels of YB328 had longer progression-free survival across several cohorts and cancer types. In contrast, those enriched with a common Bacteroidaceae member, Parabacteroides vulgatus, tended to do worse. When these microbes were moved into mice, the story held: YB328 turned PD-1 therapy into a much more potent anti-tumour tool, while P. vulgatus left tumours largely unbothered.
How one bacterium re-wires dendritic cells
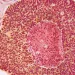
YB328 doesn’t just “boost the immune system.” It changes how key immune cells are made. In both lab and animal studies, this bacterium guides early immune precursors to develop into a specific type of dendritic cell,
(sidenote:
cDC1 dendritic cells
A specialised subset of dendritic cells skilled at presenting antigens and activating strong CD8 T-cell responses against tumours.
)
, by turning on the genes that drive this pathway. It uses a set of sensing signals inside these cells to do so.
Once formed, these gut-trained cDC1 cells don’t stay in the intestine. They move step-by-step through the lymphatic system and eventually reach the tumour. Using special mouse models that let researchers track cell movement, the study shows these gut-derived dendritic cells physically entering the tumour environment.
Inside the tumour, they present tumour antigens more effectively, activate more CD8 T cells, and help those T cells recognise a wider range of tumour targets, including weaker signals that would normally be missed. In simple terms, YB328 helps the immune system “see” more of the tumour and respond with greater strength and breadth.
Microbial competition and therapeutic imitation
Equally striking is what happens when ecology works against us. In mice colonized with a “non-responder” microbiome, adding YB328 can rescue PD-1 efficacy, but only if competing strains like P. vulgatus don’t block its
(sidenote:
Engraftment
The successful establishment and persistence of a microbial strain in the gut after it is introduced.
)
. One microbe can cancel out another’s benefit, a sobering reminder for any future live biotherapeutic strategy.
Finally, the authors show that a defined cocktail of (sidenote: TLR agonists Molecules that activate Toll-like receptors, stimulating innate immune pathways and boosting immune cell activation. ) can mimic much of YB328’s effect on cDC1 programming and PD-1 synergy. This work reframes the gut not just as a biomarker source, but as a tunable upstream regulator of dendritic cell biology and checkpoint response, opening the door to microbiome-guided or TLR-based adjuvants that might turn more of our “non-responders” into durable responders.