Interconnected pathways link plasma lipids, fecal microbiota and brain activity to cognition related to childhood malnutrition
COMMENTED ARTICLE - Children's section
By Prof. Emmanuel Mas
Gastroenterology and Nutrition Department, Children's Hospital, Toulouse, France
Comments on the original article by Portlock et al., Nat Commun 1
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Malnutrition affects more than 30 million children every year and has profound immediate and long-lasting repercussions. Children who survive often suffer long-lasting neurocognitive sequelae that impact on their school performance and socio-economic status. The mechanisms behind these consequences are poorly understood. Using SHAP models interpreted by multisystem random forest and network analysis, the authors show that moderate acute malnutrition (MAM) is associated with increased stool Rothia mucilaginosa and Streptococcus salivarius and decreased Bacteroides fragilis in a group of one-year-old children in Dhaka, Bangladesh. These changes in the microbiome form interconnected pathways involving reduced plasma levels of oddchain fatty acids, decreased electroencephalogram gamma and beta power in temporal and frontal brain regions, and reduced vocalization. These results support the hypothesis that prolonged colonization with oral commensal species delays the development of the gut and brain microbiome. Although causal links need to be validated by empirical data, this study provides useful information to improve interventions targeting neurodevelopmental deficits associated with MAM.
What do we already know about this subject
Childhood malnutrition is a major public health problem and one of the leading causes of death before the age of five. Moderate acute malnutrition (MAM) is associated with delayed neurocognitive development, but the link remains poorly understood. It is also associated with dysbiosis of the gut microbiota (GM), whose establishment is slowed and marked by enrichment in Bifidobacterium and Escherichia species. These disturbances in the gut microbiota could have an impact on cerebral development via the gut-brain axis, due to defective nutrient absorption or accumulation of toxic metabolites. This inter-organ communication could be mediated indirectly by plasma lipids, as lipids are the essential constituent of the brain and are modulated by MI metabolites such as bile acids.
What are the main insights from this study?
The study was carried out in the Mirpur region of Bangladesh, and compared 159 children with MAM with 75 well-nourished controls at 12 months of age. MAM was defined by a weight/height ratio between -2 and -3 z-scores. The MAM group was significantly associated with social-demographic factors (toilet, mode of delivery and water treatment - kettle).
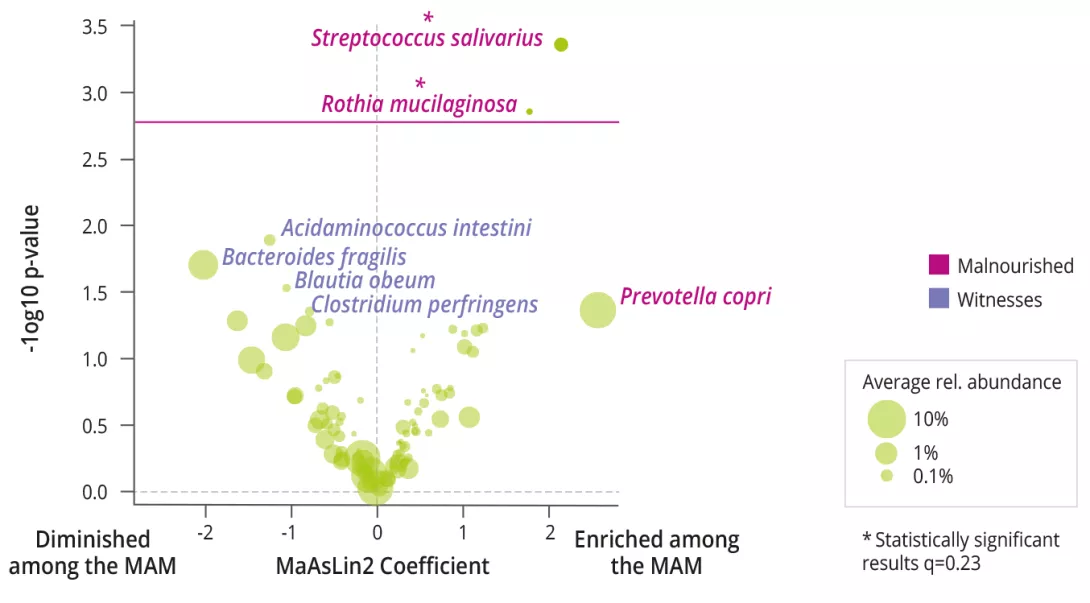
MAM was associated with decreased bacterial alpha diversity (Shannon), increased prevalence and abundance of Rothia mucilaginosa and Streptococcus salivarius (figure 1), and an increased Bacteroidetes/Firmicutes ratio. Functional analyses of the MI showed no differences.
The electroencephalogram (EEG) showed a significant decrease in beta (12-30 Hz) and gamma (30-45 Hz) frequencies in the temporal and frontal regions of children with MAM. Significant decreases in expressive communication, fine and gross motor scores, and vocalization were also observed.
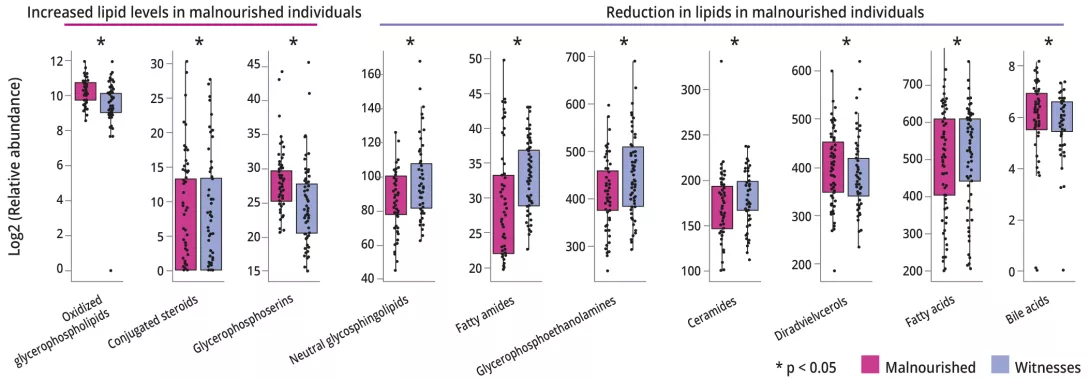
After adjusting for mode of delivery, gender and duration of exclusive breastfeeding, MAM was associated with changes in plasma lipidome, with relative abundance increased by 128 (16%) compounds and decreased by 189 (24%) (figure 2).
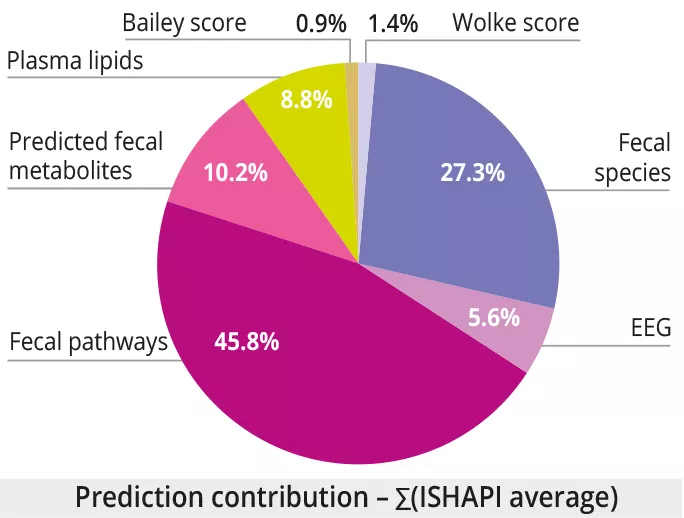
Integration of multimodal data showed that the best predictors of MAM at 12 months were: 1) plasma lipids (AUROC = 0.95 0.05); 2) brain and behavioral measures (Wolke score, EEG, Bayley score) (AUROC = 0.73±0.05, 0.71±0.10, 0.68±0.07 respectively) ; 3) the taxonomic, functional and predicted metabolite profile of the fecal microbiome (AUROC = 0.56±0.07, 0.53±0.07, 0.52±0.06). Note the high proportion of data related to the fecal microbiome for predicting MAM in multimodal analysis, despite the poor performance of the fecal microbiome (figure 3).
Multimodal network analysis predicted that a cluster of B. fragilis, pyruvate fermentation pathways, plasma ceramides, EEG and expressive communication was strongly correlated with good nutritional status at 12 months. Finally, the strongest effect as an interspecies interaction was observed between R. mucilaginosa and S.salivarius, whose combined presence amplified the prediction of MAM at 12 months.
What are the consequences in practice?
This study shows the importance of GM in the nutritional status of infants. The presence of commensal gram-positive and facultative anaerobic oral bacteria such as R. mucilaginosa and S. salivarius may be responsible for deregulation of bile acids. This could lead to lipid changes that are important for brain development.
In addition, it is important to highlight the benefit of B. fragilis in relation to fermentation pathways on nutritional status at 12 months.
- Intestinal persistence of commensal bacteria Rothia mucilaginosa and Streptococcus salivarius in MAM children overrides colonization by Bacteroides fragilis. This interferes with the synthesis of
fatty acids essential for brain development
Conclusion
This study highlights that dysbiosis of the gut microbiota is associated with abnormalities in brain development present in children with MAM, via changes in plasma lipids.

