Celiac disease: the downside of going gluten-free
Eliminating gluten is essential for managing celiac disease. But what are the effects on gut microbiota and intestinal function of following a gluten-free diet for a full year? This British study offers insights. 1
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
About this article
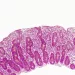
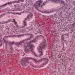
A lifelong (sidenote: Gluten Gluten (from Latin glue): a viscous nitrogenous substance formed when flour is hydrated. It originates from specific proteins – glutenins and gliadins – found in cereals, primarily wheat. ) -free diet is mandatory after a diagnosis of (sidenote: Celiac disease A disease caused by a malfunction of the immune system, which mistakenly attacks normal components of the body – in this case, the small intestine. It is triggered by the ingestion of gluten in genetically predisposed individuals. ) . Yet its effects on intestinal function and gut microbiota remain poorly understood. Hence the relevance of this observational study, which assesses the intestinal function and microbiota of 36 celiac patients before and after one year on a gluten-free diet, compared with 36 healthy controls following a standard diet.
2 to 3 As with other autoimmune diseases, celiac disease is more common in women, who are affected 2 to 3 times more frequently than men. ²
Before gluten elimination
Newly diagnosed patients not having started a gluten-free diet differed from healthy volunteers in that they showed higher levels of somatization, depression, anxiety, gastrointestinal symptoms and a 5% decrease in stool water content. The researchers also observed:
- a significantly higher water content in the small intestine (+57%), potentially due to a combination of impaired absorption (villus atrophy), increased secretion (crypt hyperplasia) and disrupted intestinal motility;
- slower intestinal transit (+83%), possibly linked to mucosal lesions, inflammation affecting motility, malabsorption and imbalances in gut hormones.
Although the team did not identify a specific gut microbiota signature for celiac disease, it did find differences in certain bacterial taxa – some of which may relate to altered intestinal function. For example, the reduced abundance of Blautia could be linked to slower transit and larger volumes of material in the colon.
95%
Genetic predisposition plays a key role in celiac disease, which is strongly associated with specific human leukocyte antigen (HLA) genes. Most CD patients (approximately 95%) express genes encoding the major histocompatibility complex (MHC) class II protein HLA-DQ2. 3
20%
The autoimmune origin of celiac disease is confirmed by the presence of serum autoantibodies and the frequent association with other autoimmune disorders, observed in 20% of patients (e.g. dermatitis herpetiformis, thyroiditis, type 1 diabetes, primary biliary cholangitis). 4
A diet that influences the microbiota
After 12 months of gluten elimination, patients reported improved well-being (less somatization, reduced anxiety, slight improvement in transit, milder symptoms, etc.), but not to levels comparable with those of healthy controls. This suggests that while gluten avoidance is essential, it is not sufficient on its own.
One year on a gluten-free diet that eliminates wheat and its fibers (resistant starch and arabinoxylan) had a mostly negative impact on the microbiota and metabolic pathways: reduced abundance of Bifidobacteria and therefore the enzymes involved in breaking down starch and arabinoxylans; and increased presence of E. coli, Enterobacter and Peptostreptococcus, leading to an increase in the associated proteolytic activity.
Imbalances persisted despite good adherence to the diet, confirmed in most patients by normalized anti-transglutaminase antibodies. This result was observed despite strong adherence by most patients to the diet as evidenced by normalized anti-transglutaminase antibodies, indicating a successful immune response.
30% of the patients showed persistent or worsening symptoms following gluten-free diet. ¹
14 Gluten-containing cereals” (wheat, rye, barley, oats, spelt, kamut, or their hybrid strains) and products made from these cereals are included on the list of 14 major allergens as defined by European food labeling regulations. ⁵
Persistent symptoms
Most notably, 1 in 3 patients reported persistent or even worsened gastrointestinal symptoms while on the gluten-free diet.
These persistent symptoms may be linked to specific alterations in the gut microbiota, independent of the immune response to gluten.
(sidenote:
Branched-chain fatty acids
Appeared to correlate with symptoms, and the persistence of symptoms was associated with microbiota composition (particularly with respect to the Bifidobacterium, Alistipes and Ruminococcus genera).
Although the gluten-free diet remains the only current treatment for celiac disease, it disrupts the microbiota and does not fully resolve symptoms. As a result, the authors suggest combining the diet with targeted prebiotics and/or synbiotics to counteract these negative effects.
)
appeared to correlate with symptoms, and the persistence of symptoms was associated with microbiota composition (particularly with respect to the Bifidobacterium, Alistipes and Ruminococcus genera).
Although the gluten-free diet remains the only current treatment for celiac disease, it disrupts the microbiota and does not fully resolve symptoms. As a result, the authors suggest combining the diet with targeted prebiotics and/or synbiotics to counteract these negative effects.
2. Malamut G, Cellier C. Place et bilan de la maladie cœliaque. Hepatogastroenterology, 2012;19:597-606.
5. EU Regulation No. 1169/2011 on the provision of food information to consumers (“INCO” Regulation)