Gut microbiota and stress-related disorders
Overview
By Pr. Sian M. J. Hemmings
Department of Psychiatry, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa
Lay public section
Find here your dedicated section
Sources
This article is based on scientific information
Sections

About this article
Stress-related disorders, including posttraumatic stress disorder (PTSD), major depressive disorder (MDD) and anxiety disorders, are common psychiatric disorders with a dysfunctional response to stress a key pathogenic mechanism. These disorders are highly complex and debilitating, and are associated with increased mortality and morbidity. There is considerable evidence implicating the role of the gut microbiota in psychiatric disorders, including stress-related disorders. Delineating a specific gut microbial profile associated with the development of these psychiatric disorders may facilitate identification of reliable biomarkers of diseaseassociated risk and predict predisposition to develop such disorders. Moreover, the gut microbiota can easily be manipulated and could, therefore, offer a simple and sustainable treatment option to alleviate symptoms of stress-related disorders. This article reviews current literature on the microbiome-gut-brain axis, and how this bidirectional system of communication may play a role in the aetiology of PTSD, MDD and anxiety disorders.
STRESS-RELATED DISORDERS
Psychiatric disorders are chronic, debilitating disorders that significantly impair daily functioning, and are among the top ten leading causes of burden of disease worldwide [1]. Exposure to environmental stressors and trauma is associated with increased incidence of post-traumatic stress disorder (PTSD), major depressive disorder (MDD) and anxiety disorders [2, 3]. These stress-related disorders are associated with increased mortality, reduced life expectancy, are highly comorbid, and exhibit variable response to first line pharmacotherapy. There are no clinically actionable biomarkers for these disorders, further complicating their diagnosis and treatment. To facilitate the development of novel therapeutic strategies and potential interventions, it is imperative that we gain deeper insight into the biological mechanisms underlying these disorders.
THE MICROBIOME-GUT-BRAIN (MGB) AXIS
“Microbiota” is the term referring to the trillions of microorganisms that live in and on us. The complete catalogue of these microbes and their genes constitutes the human microbiome. The gut microbiome, crucial in maintaining numerous aspects of our physiological functioning, is a dynamic system, the composition of which is affected by numerous factors including host genetics, age, diet and ethnicity [4-6]. The microbiome-gut-brain (MGB) axis is a complex, bidirectional system of communication between the gut microbiome, the gut, and the central nervous system (CNS), facilitated by direct and indirect communication pathways (Figure 1).
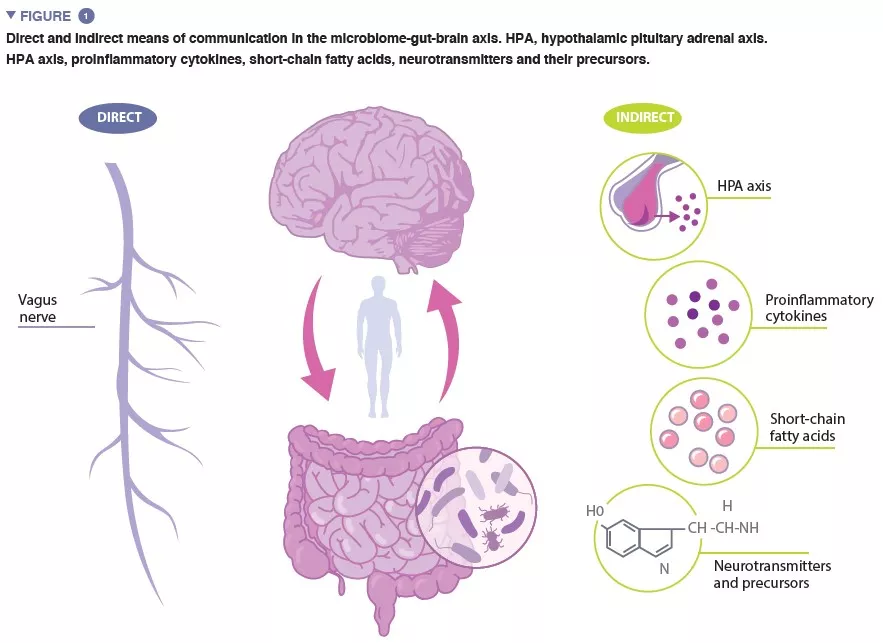
The vagus nerve, the major parasympathetic nerve of the autonomic nervous system, represents a direct link between the gut and the brain, with vagal afferents and efferents facilitating reciprocal interaction between the enteric nervous system and the brain. Indirect communication within the MGB axis takes on many forms. Microbiota produce several microbial-derived molecules, including neurotransmitters and metabolites, which act at multiple locations in the body. Many of these molecules, including serotonin (5-HT) have been found to regulate behaviour, brain function and health. As much as 95% of the 5-HT in the body is produced in the enterochromaffin cells lining the intestine, and 5-HT levels in the gut are influenced by microbial metabolites including indole, bile acids and short-chain fatty acids (SCFAs). 5-HT produced in the gut cannot bypass the blood-brain barrier (BBB), and therefore cannot affect 5-HT levels in the brain. However, animal studies provide evidence to suggest that levels of the 5-HT precursor, tryptophan, modulated by certain gut bacteria, are associated the regulation of 5-HT neurotransmission in the brain [7].
Alterations in SCFAs, a product of bacterial fermentation of host-undigestible polysaccharides, have been found to be associated with exposure to chronic stress and depressive-like behaviour in animal studies. SCFAs are involved in a number of regulatory functions, including modulation of gut activity and intestinal integrity, and activation of microglia (innate immune cells in the brain, which play an important role in regulating neuronal survival and responses). SCFAs are capable of crossing the BBB, and in doing so, may affect brain function.
It is well established that the gut microbiome plays an important role in the development of both the peripheral and central immune systems, and accumulating evidence suggests that increased inflammation is associated with stress-related disorders. An imbalance in gut microbial composition can compromise the integrity of the intestinal epithelium [8], increasing intestinal permeability and facilitating translocation of bacteria, or bacterial components, across the epithelial barrier into systemic circulation. This promotes low-grade inflammation, which stimulates increased expression of pro-inflammatory cytokines. Proinflammatory cytokines can stimulate the hypothalamic-pituitary adrenal (HPA) axis to secrete cortisol, which may further increase intestinal permeability. Indeed, evidence of gut and brain barrier dysfunction has been reported in stress-related disorders.
Systemic administration of lipopolysaccharides (LPS), a major component of the outer membrane of gram-negative bacteria, has been found to result in acute anxiety and increased depressive-like symptoms, as well as cognitive deficits, and LPS-induced increase in proinflammatory cytokine levels have been found to alter neuronal activity in limbic areas of the brain. LPS has also been found to induce increased cytokine production in the CNS, compromising the integrity of the BBB, resulting in a “leaky brain”.
INVESTIGATING THE GUT MICROBIOME IN STRESS-RELATED DISORDERS: PRECLINICAL AND CLINICAL FINDINGS
Several preclinical investigations support the idea that the gut microbiome composition is associated with stress-related disorders. Investigations using germ-free (GF, microbiologically sterile) animals have played a crucial role in our understanding of the MGB axis. In their seminal investigation, Sudo and colleagues [9] observed an exaggerated stress response, evidenced by increased levels of corticosterone, in GF mice compared to controls, following acute restraint stress. This exaggerated HPA axis response to stress was normalised upon mono-colonisation of the GF mice with Bifidobacterium infantum. Studies have also shown that it is possible to transfer anxiety- like behavioural phenotypes between two mouse strains, by means of fecal microbiota transplant (FMT) [10]. Similarly, several studies have reported on the development of depressive- and anxiety-like behaviour, and altered neuroendocrine-immune pathways, in microbiota-depleted rodents following FMT from humans diagnosed with MDD, suggesting a causal role for gut microbiota in depressive-like behaviour [11-13]. Animal studies have also shown that exposure to stress can cause long-lasting alterations in the gut microbiome – two recent studies reported on decreased relative abundance of Akkermansia muciniphila in the gut microbiome of stressed animals over time, compared to control animals [14, 15]. A. muciniphila and the outer membrane coat of the bacteria (Amuc_1100) have been found to ameliorate depressive-like behaviour, and increase circulatory levels of 5-HT.
Comparatively few clinical studies have been conducted to determine the association between the gut microbiome and stress-related disorders. Thus far, the only published data on the gut microbiome in PTSD emanates from our research group [16], where a consortium of four bacterial genera was found to predict PTSD status with 66.4% accuracy. In addition, MDD diagnosis in the sample was found to be associated with increased relative abundance of the phylum Bacteroidetes. Other studies indicate that bacterial taxa associated with both depression and anxiety disorders are characterised by a higher relative abundance of taxa that induce a proinflammatory environment and a reduced abundance of SCFA-producing bacteria [17].
This field of research is, however, still in its infancy, currently limited by the lack of standardisation in gut microbiome analysis, from sample collection to the analytical pipeline. In many cases, factors that may confound results, including diet, medication use, ethnicity and host genetics, were not accounted for in the studies reviewed above. Moreover, most of the studies conducted have been cross-sectional in design, limiting our ability to disentangle cause from consequence, and very few have investigated potential mechanisms underlying the associations.
A. muciniphila is a gram-negative anaerobic bacterium, found primarily in the intestinal mucosa. It plays a role in maintenance of intestinal barrier integrity as well as in immune and metabolic regulation.
MODULATION OF THE MGB AXIS: PROBIOTICS
The gut microbiome is tractable and has the potential to be modulated, making the search for gut microbiome markers associated with stress-related disorders particularly attractive. Probiotics are defined as living microorganisms which, when administered in adequate amounts, confer a health benefit on the host; psychobiotics refer to probiotics which confer a benefit on mental health, cognition and behaviour. Recent publications have indicated moderate beneficial effects of psychobiotics in alleviating depressive and anxiety symptoms in both healthy and clinically-defined cohorts [18]. It is, however, important to remain cautious when interpreting results from current studies, as they are variable with regards to probiotic formulation and dosage, sample characteristics (clinical phenotype and severity of depression/anxiety), and follow-up time. In addition, the benefit of psychobiotics over, and interactions with, antidepressant medication has not yet been extensively investigated, although some intriguing results from preclinical studies suggest that certain probiotics, when administered in multi-strain format, possess antidepressant effects similar to, and sometimes with larger effect than, current first-line antidepressants [19]. Such psychobiotics, when used in conjunction with antidepressants, may have particular use in individuals with treatment-resistant depression.
Conclusion
Evidence to suggest that the gut microbiome is altered in stress-related disorders continues to grow, and while much work remains to be done in the field, delineating a specific gut microbial profile associated with the development of stress-related disorders may facilitate identification of reliable biomarkers of disease-associated risk and predict predisposition to develop these disorders. The gut microbiome can easily be manipulated and could, therefore, offer a simple and sustainable treatment option to alleviate symptoms of PTSD, MDD and anxiety disorders.
• 1. GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022; 9: 137-50.
• 2. van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 891-907.
• 3. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 2010; 35: 169-91.
• 4. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691-6.
• 5. Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014; 5: 3564.
• 6. Ayeni FA, Biagi E, Rampelli S, et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep 2018; 23: 3056-67.
• 7. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry 2013; 18: 666-73.
• 8. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010; 170: 1179-88.
• 9. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004; 558: 263-75.
• 10. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141: 599-609.
• 11. Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016; 82: 109-18.
• 12. Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat 2020; 16: 859-69.
• 13. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016; 21: 786-96.
• 14. Hoke A, Chakraborty N, Gautam A, Hammamieh R, Jett M. Acute and delayed effects of stress eliciting post-traumatic stress-like disorder differentially alters fecal microbiota composition in a male mouse model. Front Cell Infect Microbiol 2022; 12: 810815.
• 15. Pascual Cuadrado D, Todorov H, Lerner R, et al. Long-term molecular differences between resilient and susceptible mice after a single traumatic exposure. Br J Pharmacol 2022; 179: 4161-80.
• 16. Malan-Muller S, Valles-Colomer M, Foxx CL, et al. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur Neuropsychopharmacol 2022; 56: 24-38.
• 17. Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev 2021;83: 101943.
• 18. Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, Müller DJ. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int J Mol Sciences 2022; 23: 4494.
• 19. Ra Y, Eu P, Ev V, Mv O, Mv M, Gi K, et al. A Multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics and Antimicrobial Proteins 2020; 12: 973-9.