Hyperglycemia, hyperlipidemia, hypertension: the effects of a diet that is too high in fat are known but they are only the tip of the iceberg. Researchers have thoroughly disclosed the major role of the intestinal microbiota in these metabolic disruptions. They also sorted the good fats from the bad
The same observation is made in laboratory mice given a high fat feed as in patients with metabolic syndrome: their intestinal flora does not resemble that of their healthy counterparts: too much fat on a daily basis reduces the amount of Akkermansia muciniphila, a beneficial bacterium which improves glycemia and insulin sensitivity, and protects against the formation of fatty plaques in the vessels (atherosclerosis). As its name indicates, this bacterium also produces a substance called “mucin”, which strengthens the protective mucus of the intestinal barrier. Another side-effect of excess dietary fat: lactobacilli and bifidobacteria, “good” bacteria which reduce inflammation and formation of adipose tissue, are decreased.
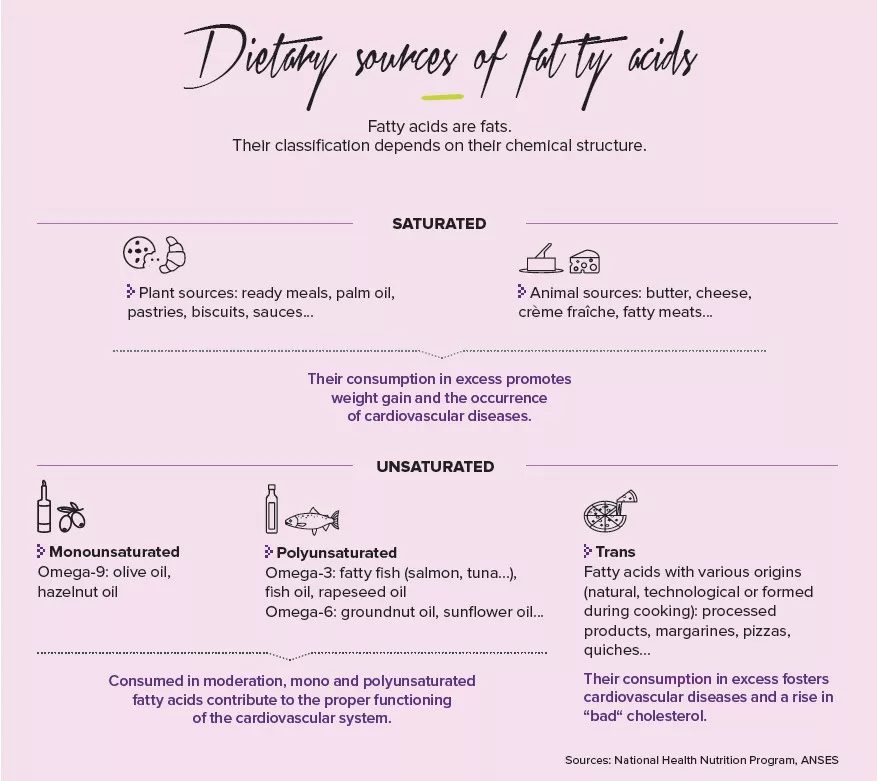
Not all fats are the same
But incidentally, which fats are we talking about? Saturated fatty acids like palm oil are indeed to be avoided, as is hammered home by public health messages: they are associated with lowered bacterial diversity and weight gain. Conversely, the oleic acid contained in olive oil, a monounsaturated fatty acid of the omega-9 family, is thought capable of restoring bacterial diversity and of reducing weight –in mice at least. We should also rely on omega-3 polyunsaturated fatty acids, such as fish oil, which promote the presence of Akkermansia muciniphila, lactobacilli and bifidobacteria. These omega-3-containing foods should moreover take precedence over those containing omega-6, which are also essential to the organism but are to be consumed with moderation as they cause inflammation and a reduction in bifidobacteria
“First and foremost, eat your fiber”
And as fat doesn’t do everything, good or bad, another food category also carries weight with respect to metabolism: fibers. Those indigestible sugars present in cereals, tubers, nuts, seeds, fruits and vegetables. Without fibers to ferment to extract their energy in the form of SCFA, bacteria begin to snack on the protective mucus which lines our intestinal cells, exposing them to bacterial invasion. Moreover, fibers enable better control of glycemia, probably through the presence of Prevotella in our intestines. Conclusion: for your microbiota, eat fat without excess–but good fat–and don’t forget your fibers!