Menopause-induced changes lower gut microbiome diversity and cause a shift toward greater similarity to the male gut microbiome. This review details the healthrelated consequences of these changes. During perimenopause, a gradual decline in hormone levels disrupts the gut microbiome balance and contributes to adverse health outcomes, including cardiometabolic disease and changes in oestrogen metabolism. Hormonal fluctuations during menopause change the oral microbiome, heightening the risk for dental caries, periodontitis, and oral infections such as candidiasis. Menopauseinduced vaginal microbiota alterations increase susceptibility to bacterial vaginosis, vulvovaginal atrophy, and recurrent urinary tract infections. Menopause also alters the diversity and abundance of gut microbiota that have been linked to inflammation. Chronic dysbiosis-induced inflammation predisposes menopausal women to metabolic disorders and autoimmune diseases.
This article bridges the gap between endocrinology and microbiology, and emphasises the systemic impact of menopause beyond reproductive health. A key strength of the review is its holistic examination of menopausal-related hormonal fluctuations with corresponding shifts in gut and vaginal microbial composition and diversity. This opens the door to exploring microbiome-based biomarkers for managing menopausal symptoms such as genitourinary syndrome, metabolic changes, or inflammation. This article’s interpretation of age-related changes in women’s health enriches the growing interest in the human microbiome’s role in disease. While hormone replacement therapy has shown promise in mitigating some of the adverse effects of oestrogen deficiency, its broader application is limited by its systemic risks. The targeted use of specific probiotics to restore gut microbial balance, coupled with dietary and lifestyle modifications, may offer safer, more individualised alternatives that mitigate adverse health effects of menopause.
Menopausal microbiome research is overrepresented with data from Western populations and a lack of detailed mechanistic insights. Since diet, lifestyle, and environmental factors significantly influence the microbiome, we need ethnically and geographically diverse research incorporating advanced “omics” approaches to fully elucidate these influences. More effective, personalised treatment strategies will then emerge that can improve the quality of life for menopausal women.
In conclusion, menopause is a whole-body transition involving significant changes in the microbial ecosystem. Understanding and addressing these changes can enhance patient outcomes and promote healthier ageing in women.

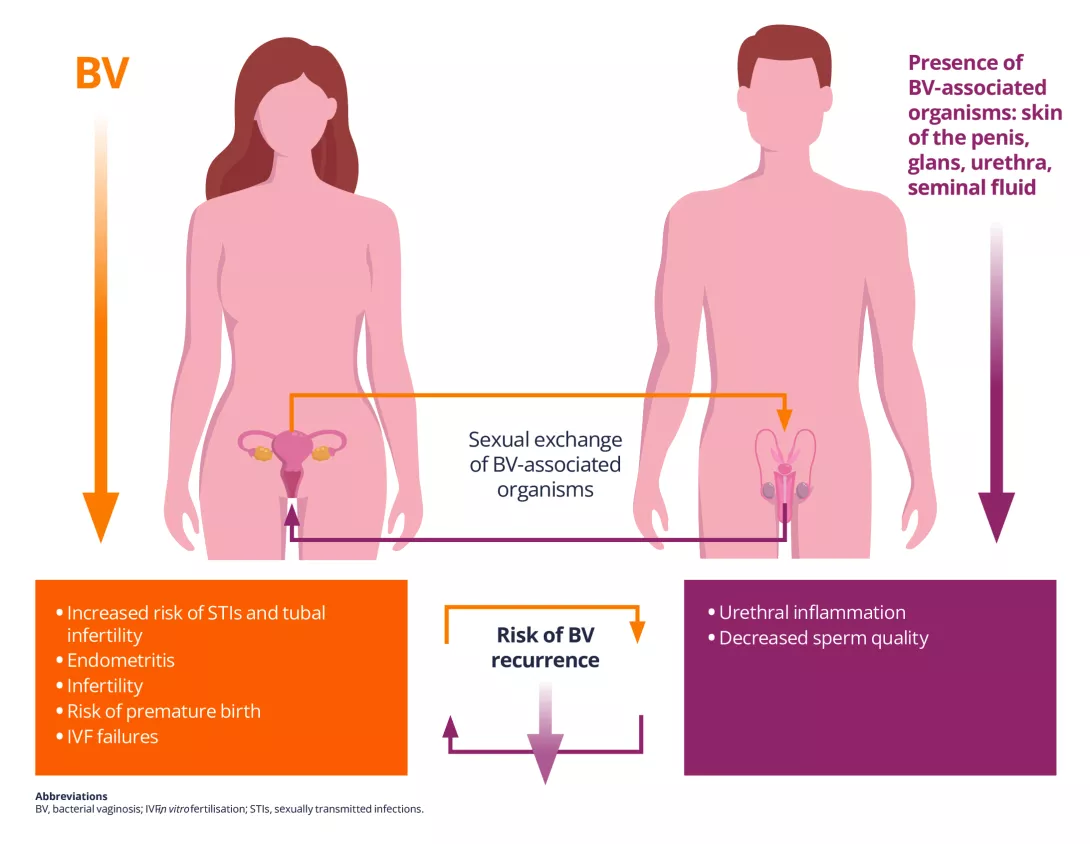
 Infertility: vaginal bacteria and viruses both implicated
Infertility: vaginal bacteria and viruses both implicated
 Periods & vaginal microbiota: Science in progress…
Periods & vaginal microbiota: Science in progress…