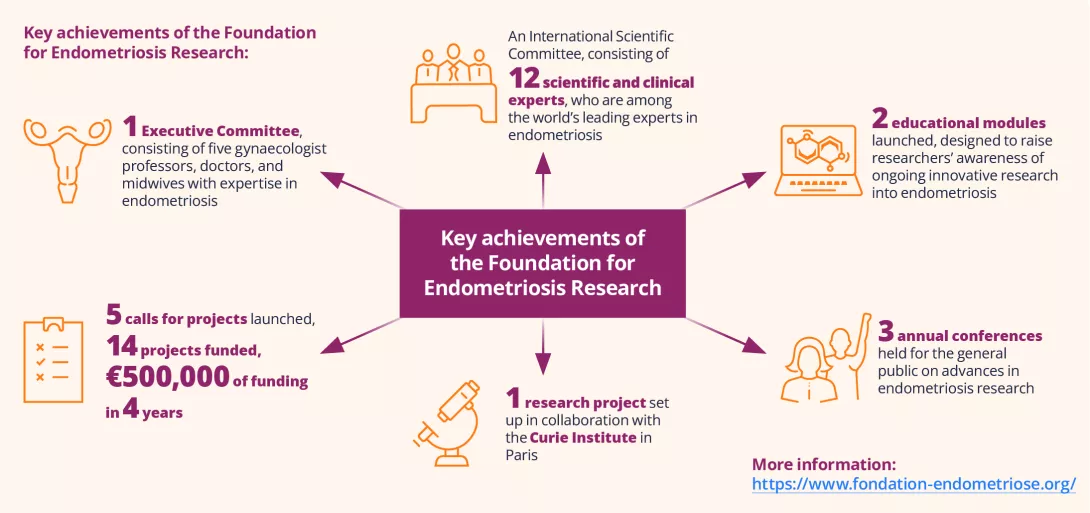
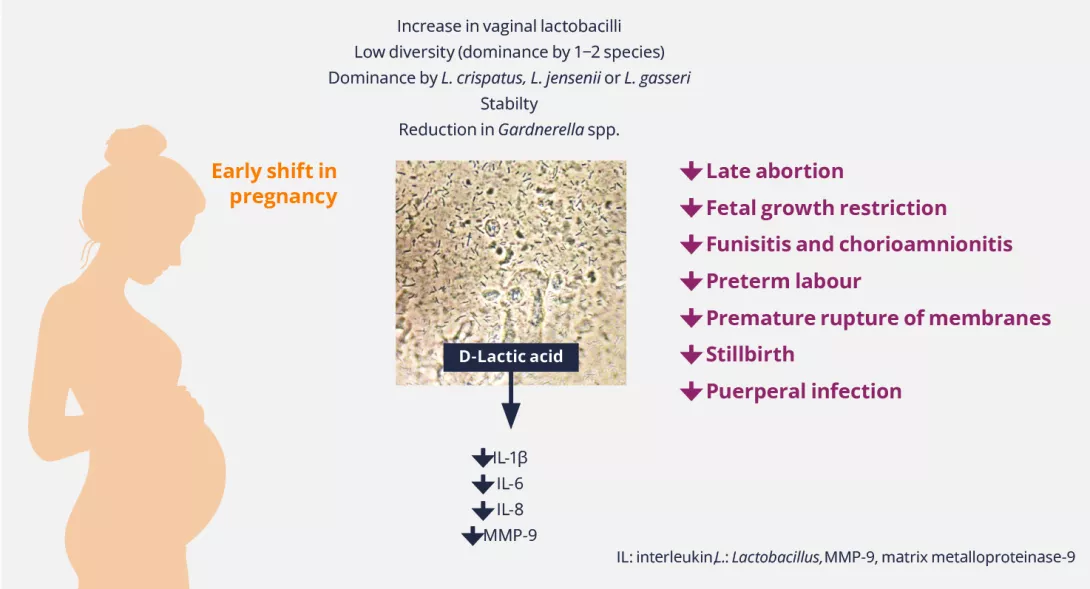
What is the prevalence of endometriosis?
A.H.: Endometriosis is surprisingly common – as common as asthma and diabetes. It affects an estimated 1 in 10 women.
W.F.: In my practice of irritable bowel syndrome (IBS), it’s even more common – certainly more than 25%.
What are the signs and questions to ask to avoid missing a diagnosis?
A.H.: Signs are varied and diagnosis can be difficult. The main symptom is chronic pelvic pain, which can often be debilitating, disrupting life and work. But, patients can also present with painful sex, chronic fatigue, diarrhoea and/or constipation and urinary symptoms. Any cyclical symptom can be a red flag for endometriosis.
W.F.: Increased peristalsis and softer stools during menstruation are normal, but significant and cyclical diarrhoea may not be so. Pain is expected, but not to the point of being bedridden.
A.H.: Another symptom that causes alarm is infertility. But I reassure patients with endometriosis: two-thirds of them won’t have trouble getting pregnant, and those who do generally respond well to surgery or IVF.
W.F.: I would add that endometriosis is chronic, but that doesn’t mean it’s untreatable. It’s important that any specialist involved with the care of these patients reinforces that message.
How common are digestive symptoms in women with endometriosis?
A.H.: The true prevalence isn’t known, but nearly all my patients have digestive symptoms – bloating, bowel changes, heartburn. Lesions on the bowel wall explain some symptoms, but many have superficial peritoneal disease, making the link harder to define.
W.F.: I have observed similarly, and would state that inflammatory bowel disease (IBD) is four times more common in women with endometriosis compared with the general population (4% vs 1%). IBD and endometriosis are both autoimmune conditions; having one increases the risk of the other.
Is there a need for multidisciplinary management?
A.H.: Endometriosis is a systemic inflammatory disorder. As gynaecologists, we’re not equipped to manage digestive symptoms. In Edinburgh, I’ve recently set up a joint gynaecology–gastroenterology clinic.
W.F.: When the abdominal pain strictly relates to menstruation, the gastroenterologist may find it difficult to add much. When the relationship is more loosely defined, we should investigate. Persistent digestive symptoms despite treatment may signal coexisting IBS. Also, be mindful of medications, particularly nonsteroidal anti-inflammatory drugs (NSAIDs). Occasional use is fine in young patients, but chronic use may require a proton pump inhibitor (PPI), which can cause dysbiosis. There’s no universal rule — we must tailor care to each patient.
Are gut and vaginal microbiota involved?
A.H.: There’s growing interest in the role of the gut and vaginal microbiomes in endometriosis. Some studies suggest associations, but they’re small and flawed. We need large cohort studies. I believe the microbiome plays a role, but it’s still unclear as to which comes first — microbiome changes or endometriosis. If microbiota drive symptoms, this could open the path to new treatments.
W.F.: It’s an exciting field. In patients with endometriosis, we observe gut dysbiosis with reduced short-chain fatty acids like acetate, propionate and butyrate that protect gut permeability. The same pattern can be seen in other gastroenterological conditions, like IBS or IBD, but we don’t yet understand the relationship. Maybe one day, we’ll personalise care by restoring exact missing strains. For now, we don’t know what causes what, so mechanistic studies are needed.
Should we recommend specific diets to patients with endometriosis?
W.F.: There’s no universal diet for endometriosis and we shouldn’t offer false hope. Allergies, lactose intolerance, and coeliac disease might be involved. The best step is to refer patients to a nutritionist.
A.H.: No specific “endometriosis diet” exists, but many patients report symptom relief after dietary changes. In my clinic, patients work with a dietitian to carefully adjust their diets. In our international survey of 2,500 patients with endometriosis, some found relief by stopping consumption of alcohol and caffeine, or foods containing gluten. However, without guidance, dietary restriction can be harmful.