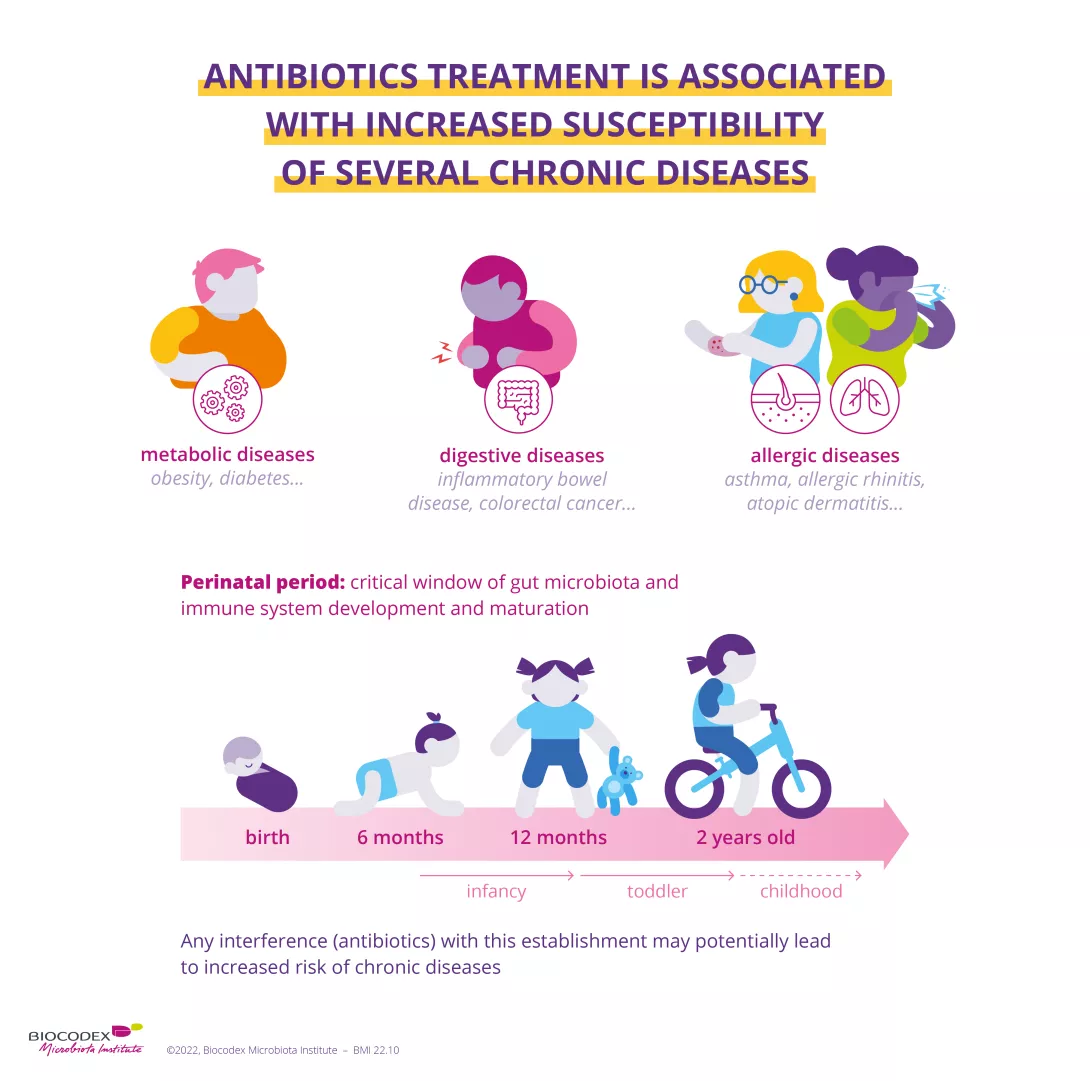
Metabolic syndrome is not, strictly speaking, a disease; its definition is the existence, in a single person, of abdominal obesity (waist measurement > 94 cm for men, 80 cm for women), associated with at least two of the following problems: abnormally elevated insulin level, hypertriglyceridemia, high blood pressure, hyperglycemia, or low HDL cholesterol (“good cholesterol”).
France spared
With 14 to 21% of people affected, compared with a quarter of Americans and almost 46% of Greeks, France has been somewhat spared the “epidemic” of metabolic syndrome. But this situation cannot last.
Unhealthy lifestyle, the primary risk factor
Other than likely genetic predispositions, it’s an unhealthy lifestyle that leads to metabolic syndrome. Junk food, paired with insufficient physical activity, causes metabolic dysfunction that leads to chronic inflammation, which itself causes metabolic disorders. It starts a vicious cycle, in which one contributor is imbalance in the microbiota or dysbiosis.
No visible signs
Other than obesity, metabolic syndrome has no visible signs since, once symptoms appear, it means that the syndrome has changed into a disease: type 2 diabetes, atherosclerosis, cardiovascular disease, etc.
Eat better, move more
For the time being, there is no treatment for metabolic syndrome. The only medical advice that works equally well as a prevention and a cure is a balanced diet with an emphasis on low glycemic index foods and regular and sustained physical activity. If the idea that probiotics and prebiotics as regulators of diet and weight is confirmed, it could also be factored into treatment for metabolic syndrome.

 The role of intestinal microbiota in respiratory infections
The role of intestinal microbiota in respiratory infections