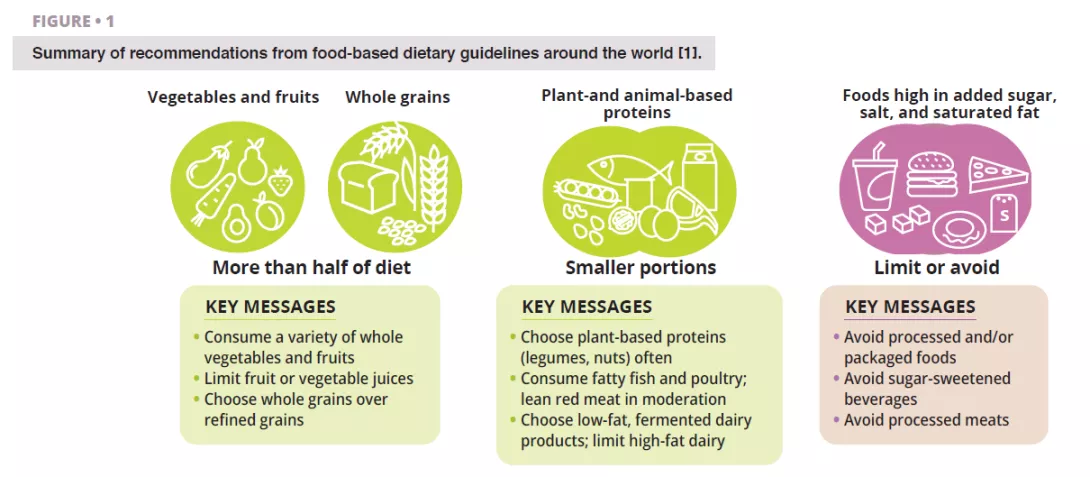
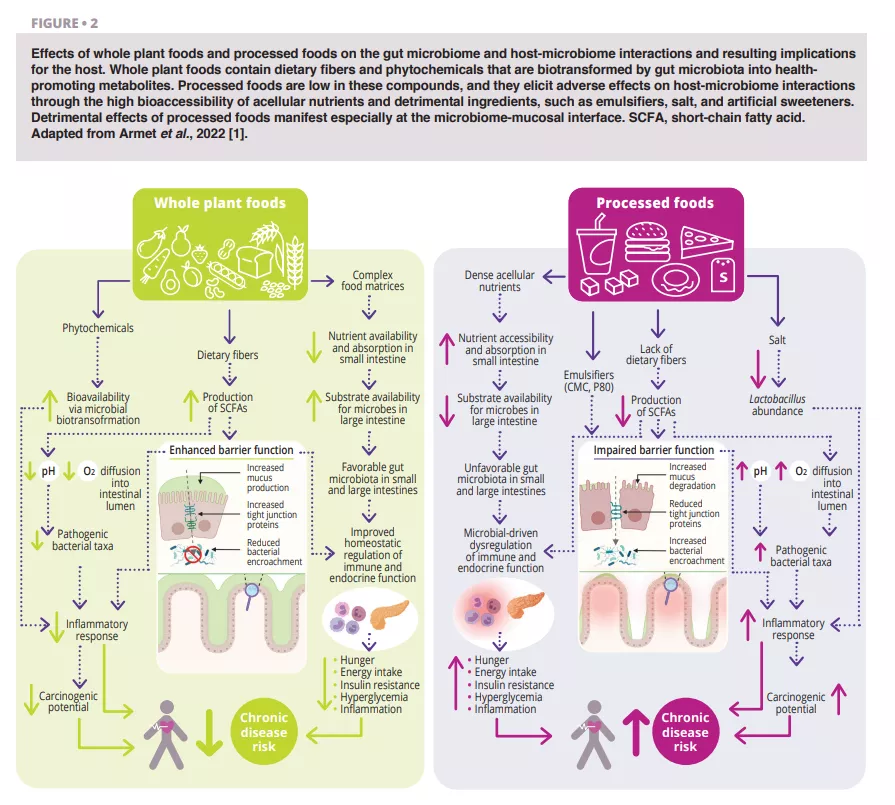
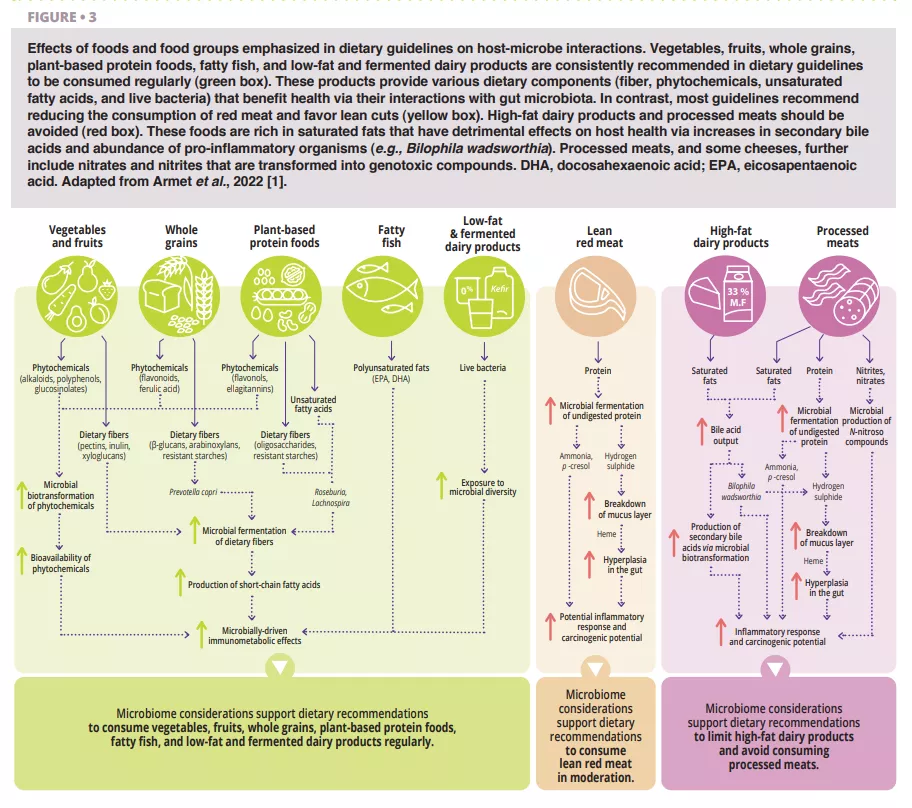
Vaginal Microbiota #20
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland
21% Only 1 in 5 women say they know exactly the meaning of the term “vaginal microbiota”
Recurrent miscarriage: a case study on vaginal microbiota transplantation (VMT)
This case has clear scientific interest. A woman with a history of late pregnancy losses and severe vaginal dysbiosis undergoes a vaginal microbiota transplant (VMT). Five months later, she becomes pregnant, with a healthy vaginal flora, and subsequently gives birth to a fullterm baby. However, the limitations of the study should be noted: it involved a single patient diagnosed with antiphospholipid antibody syndrome (APLS, a thrombophilia associated with miscarriages).
Furthermore, therapy received for this APLS during her last pregnancy may explain the results in part or in full. Prior to the transplant, the 30-year-old mother of one had suffered a series of pregnancy losses, some of them late (gestational week 27 in 2019, and weeks 17 and 23 in 2020). For the previous nine years, she had complained of itching and vaginal discharge, which worsened during her pregnancy attempts, despite treatment. With good reason: in July 2021, her vaginal microbiota showed a very strong dysbiosis, with a 91.3% dominance of Gardnerella spp. According to a compassionate use protocol, a VMT from a healthy donor was performed in September 2021, on day 10 of her menstrual cycle, without antibiotic pretreatment. While orally or vaginally administered antibiotics (metronidazole or clindamycin) can have a cure rate for vaginal dysbiosis of 80%-90% one month after treatment, the recurrence rate can be as high as 60% after one year, with the added risk of resistance.The VMT rapidly corrected the dysbiosis and its symptoms and for several months Lactobacillus strains similar to those of the donor became dominant. In February 2022, the patient became pregnant naturally. She received therapy for her APLS during this pregnancy. Regular monitoring of her vaginal microbiota revealed the return of Gardnerella spp. (41.8%) at gestational week six. A second VMT was initially planned for two weeks later, but by the day in question, L. crispatus had once again come to dominate the patient’s microbiota.
At the end of the pregnancy, a perfectly healthy baby boy was born via planned cesarean section. Although they need to be confirmed by further clinical studies, these results suggest that VMT may serve as a treatment for patients with severe vaginal dysbiosis, including those at risk of complications following in vitro fertilization.
For the authors, this case study serves as a proof of concept, but it also offers hope for therapies based on the modulation of the vaginal microbiota.