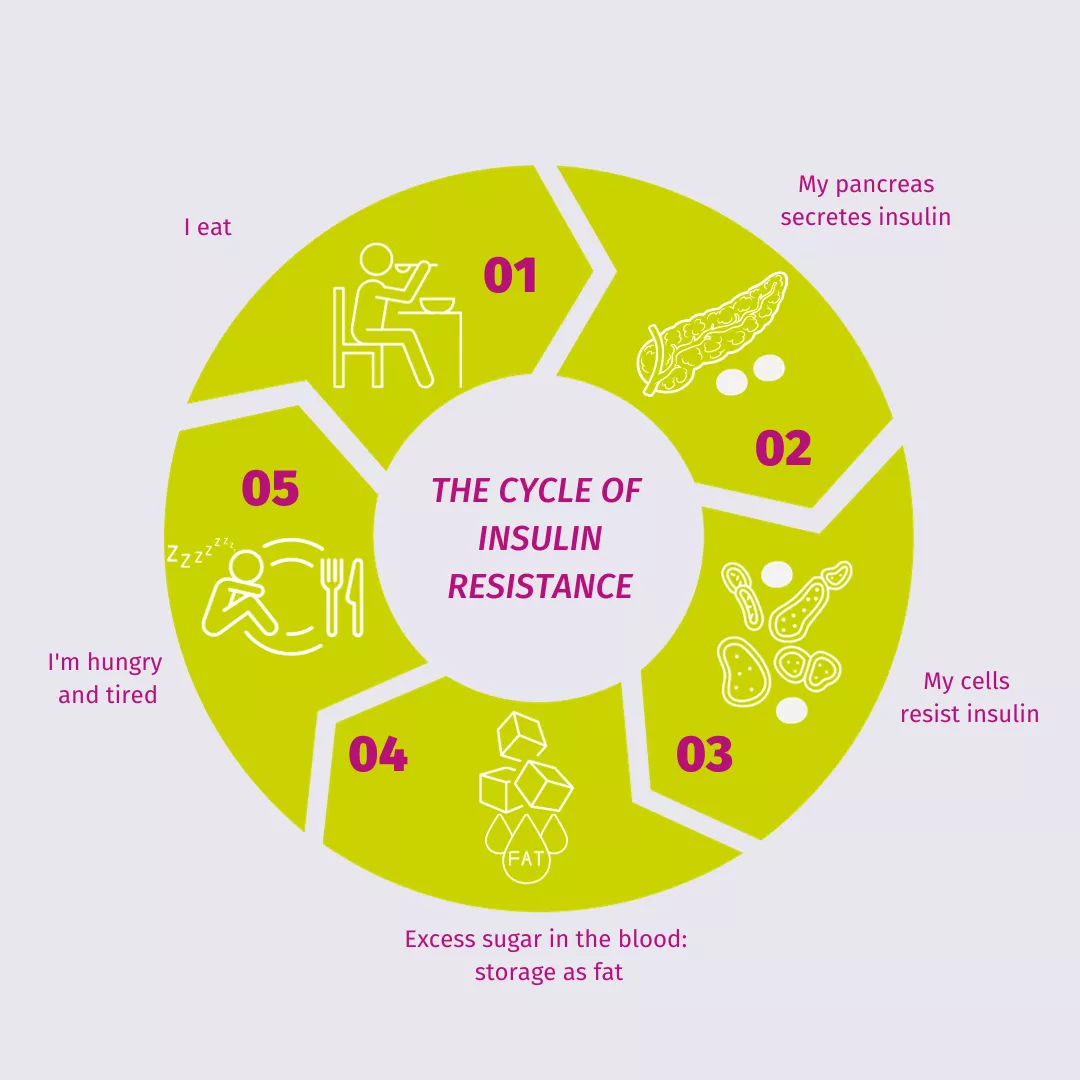
According to the International Microbiota Observatory, more than 9 out of 10 (95%) people who repeatedly benefited from information from their healthcare professional had adopted specific behaviors to maintain a balanced microbiota. What about antibiotics? Well, according to the same study, only 1 in 3 patients said their healthcare professional had informed them that taking antibiotics could upset their microbiota balance. Because information provided by healthcare professionals is a game-changing vector of behavior, during the WAAW 2024 (18-24th November), the Biocodex Microbiota Institute sheds the light on the key role played by physician regarding information about the impact of antibiotics on microbiota and health.
Train the physicians: pass it on to their patients.
Engaging healthcare professionals (antibiotics prescribers) with tailor-made content. During the WAAW campaign, Biocodex Microbiota Institute federates its physicians ‘community with two “hub” pages gathering all physicians’ tools (thematic folder, accredited training on dysbiosis and the impact of antibiotics, infographics to share with their patients, news, experts ‘interviews…).
Raising awareness of the impact of antibiotics on microbiota remains important. This two “hub” pages aim to provide physicians quick and ready-to-use material to improve their patients understanding of the importance of using antibiotics prudently.
Educate the lay public: you can take action!
Hailed as one of the greatest medical advances of the 20th century, antibiotics have saved millions of lives. Today, they pose serious public health challenges: their excessive and inappropriate use leads to the emergence of numerous resistances, which, in the long term, could eventually make them ineffective. Moreover, they also can damage the microbiota by inducing a dysbiosis. For the lay public, the Institute investigates this ambivalence role with one “hub” page gathering all content on the impact of antibiotics on microbiota.
From subsidiaries to the Biocodex Microbiota Institute passing through Beauvais manufacturing site, all Biocodex’s collaborators get involved!
For the WAAW campaign 2023, Biocodex Microbiota Institute website homepage, X, Facebook and LinkedIn accounts have turned blue. The Institute’s social networks are not the only ones… the Biocodex 9-hectare manufacturing and logistics center in Beauvais, near Paris, joined the color campaign by wearing blue during WAAW event. More than 30 spotlights have been placed to offer visitors and Biocodex collaborators a blue scenography. From November 18 to 24, the Institute invites Biocodex’s collaborators all over the world to join the campaign on social media thanks to a blue stamp they can put on LinkedIn.
#GoBlueForAMR.
 Is vaginal microbiota transfer the new miracle for C-section babies?
Is vaginal microbiota transfer the new miracle for C-section babies?