Long considered sterile, the uterine cavity is in fact home to a microbiota composed of bacteria. Although 100 to 10,000 times less numerous than the bacteria present in the vagina, uterine bacteria appear to be just as involved in reproductive health. So suggests a multicenter (13 centers located in Europe, America, and Asia), prospective, observational study that analyzed the composition of the endometrial microbiota of 342 infertile women taking part in IVF programs.
Double sampling of endometrial microbiota
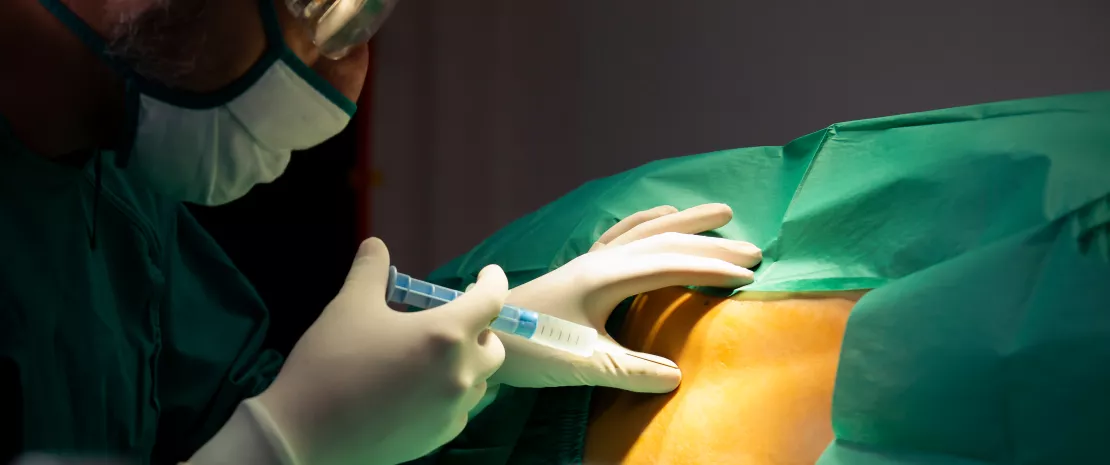
Two samples were taken prior to embryo transfer to evaluate the composition of the endometrial microbiota: the endometrial fluid was aspirated from the uterine cavity and the endometrial mucosa was sampled via biopsy. The researchers then studied the relationship between the composition of the endometrial microbiota, analyzed using 16S RNA sequencing, and the outcome of IVF, namely pregnancy carried to term (41% of patients), biochemical pregnancy (8%), miscarriage (8%), or no pregnancy (42%).
Endometrial dysbiosis associated with IVF failure
The researchers observed an increased abundance of Lactobacillus (in fluid and mucosal samples) in patients who carried a pregnancy to term. Conversely, Lactobacillus depletion and the presence of specific pathogenic bacteria, such as Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus, and Streptococcus, were associated with IVF failure or a pregnancy that did not result in a viable birth. Notably, Gardnerella and Klebsiella were over-represented in both the endometrial fluid and endometrial mucosa of patients whose IVF was unsuccessful.
Lactobacilli, a bulwark against pathogens?
These data point to the role of the endometrial microbiota in the success or failure of embryo implantation and/or the outcome of IVF. The researchers suggest that the outcome of IVF is influenced by the absence of pathogenic bacteria in the endometrium, rather than the presence of beneficial bacteria (such as lactobacilli). Lactobacillus may thus inhibit the colonization of the uterine cavity by pathogenic bacteria. However, further studies are needed to clarify the mechanisms by which these pathogenic bacteria work.
Recommended by our community