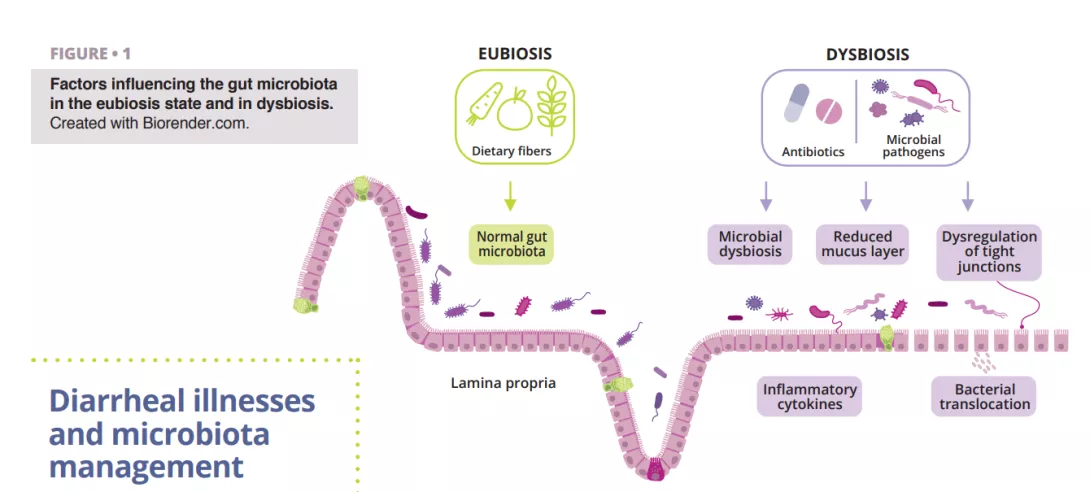
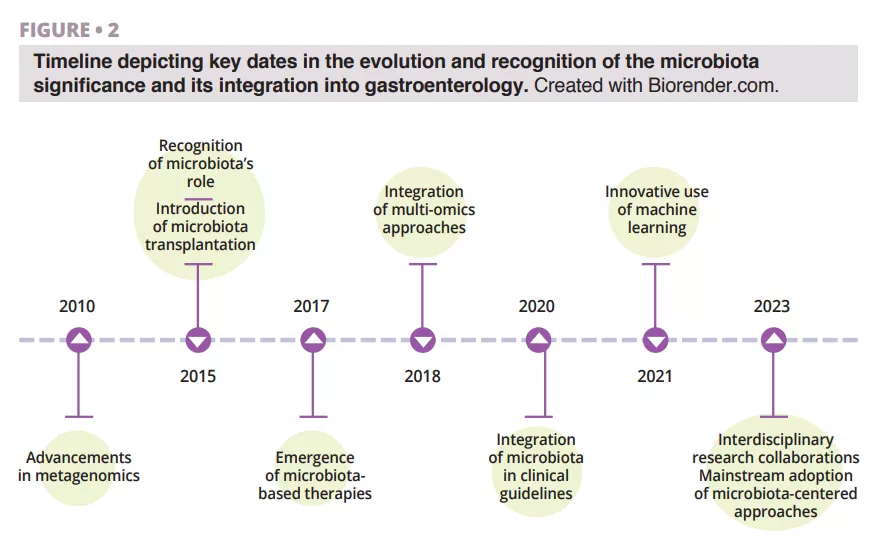
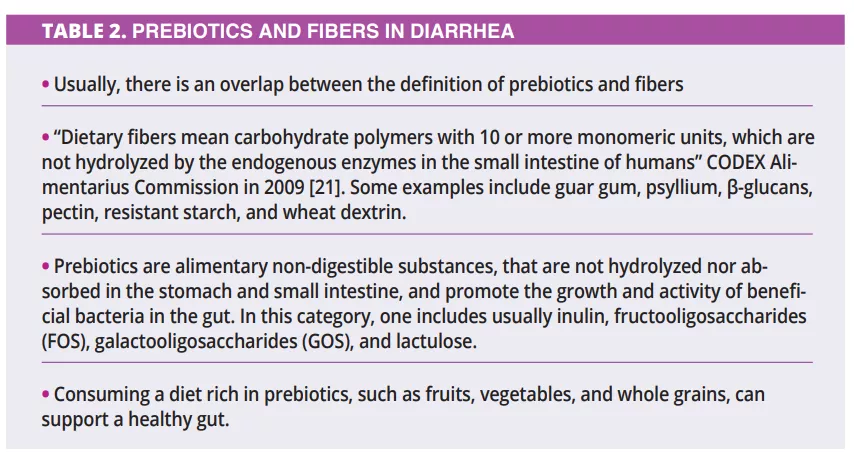
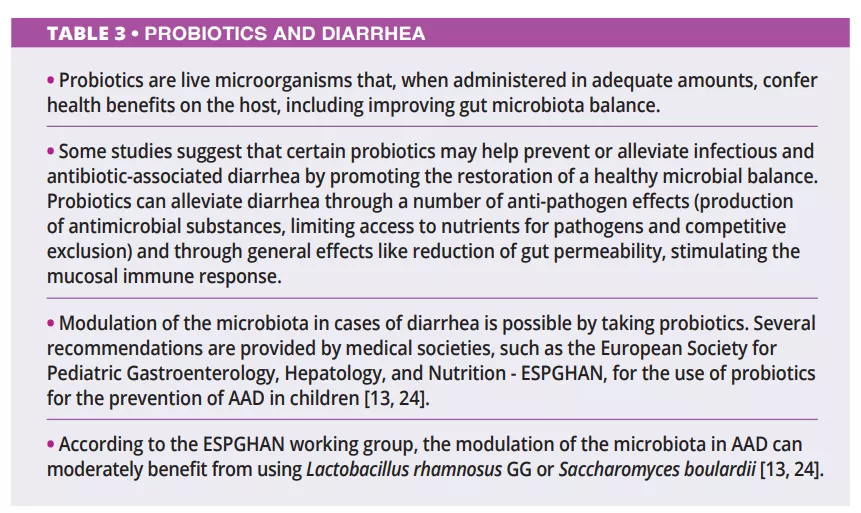
What do we already know about this subject?
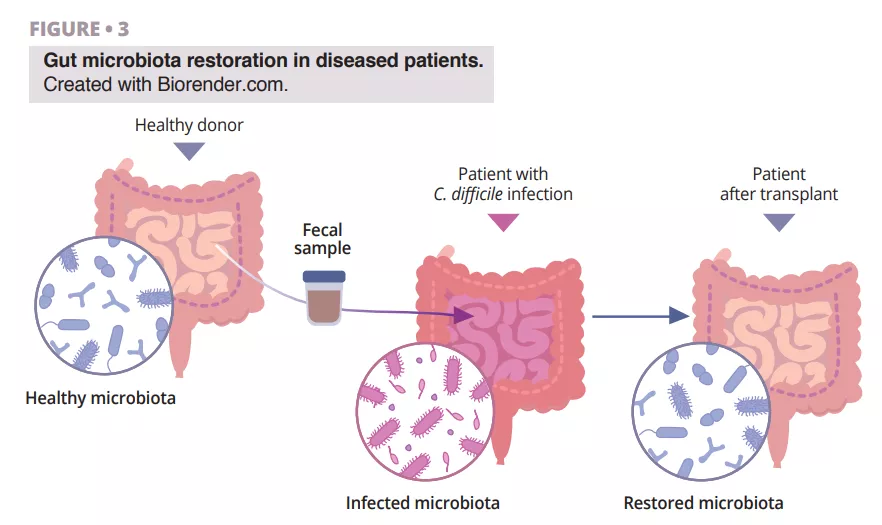
Almost half of patients with advanced melanoma receiving anti-PD-1 monotherapy develop primary resistance, highlighting the need to develop new therapeutic strategies to improve the response to immune checkpoint inhibitors (ICIs). Although the combination of anti-PD-1 and anti-CTLA4 (cytotoxic T lymphocyte-associated antigen-4) increases the response rate, this therapy is limited by the high number of immune-related adverse events (IR-AEs). The gut microbiome has emerged as an essential regulator of local and systemic immune responses. Several studies in cancer patients treated with ICIs have shown that specific gut bacteria are associated with both immune system response and adverse events [1]. More specifically, the presence of certain commensal genera, such as Ruminococcus, Faecalibacterium and Eubacterium, has been associated with positive outcomes in melanoma patients [2]. The therapeutic potential of the gut microbiome was first demonstrated in mouse models combining ICIs with FMT using faeces from non-responder (NR) patients who were associated with ICI resistance [1]. Two studies showed that FMT in patients with a long-term response to ICI therapy circumvented anti-PD-1 resistance in almost 30% of patients with ICI–refractory melanoma [3, 4]. In these studies, the microbiota of patients changed after FMT, and an increase in Ruminococcaceae and Bifidobacteriaceae was observed in responder (R) patients plus a reprogramming of the tumour microenvironment with increased CD8+ T-cell infiltration and interferon-γ signalling. These clinical findings confirm the potential of microbiome-based interventions to overcome ICI resistance in melanoma.
What are the main insights from this study?
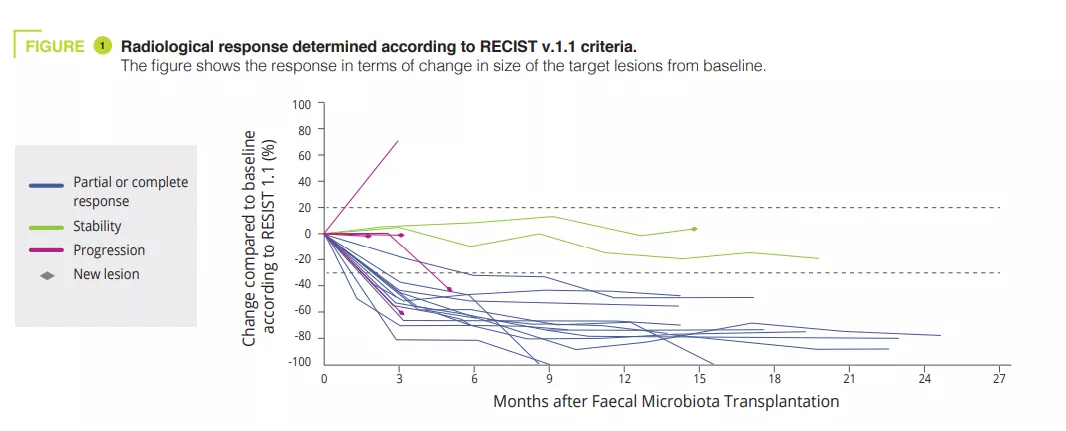
In this article, the authors reported the clinical and translational findings from a Phase I trial (NCT03772899) combining FMT from healthy donors with the PD-1 inhibitors nivolumab or pembrolizumab in treatment-naive patients with advanced melanoma (figure 1). The toxicity observed (85% IR-AEs, of which 25% grade 3 toxicity and zero grade 4 or 5 toxicity) was similar to that reported in the Phase III trials for anti-PD-1. The observed clinical efficacy (objective response 65%) was higher to that of nivolumab and pembrolizumab monotherapy in Phase III trials (objective response 42-45%) and in real-world data (objective response 17.2-51.6%). However, the absence of a control arm and the small size of the study hindered the interpretation of the results.
Unlike the previous studies [3, 4], it included patients receiving first-line treatment, a single FMT was performed by oral capsule, donors were healthy subjects (and not ICI responders) and, finally, only PEG (without the use of antibiotics) was used for the preparation. By studying the microbiota of donors and recipients, the authors observed that the microbiota of responders was enriched in Ruminococcus SGB15234 and SGB15229, Alistipes communis, Eubacterium ramuleus and Faecalibacterium SGB15346, while the abundance of Enterocloster aldensis and Enterocloster clostridioformis decreased. In previous studies, the increase in Faecalibacterium was also associated with the response to ICI [3, 4].
The authors then experimented on mice colonised with human microbiota and observed a similar efficacy of the faecal transplantation from healthy subjects in this context, with an effect associated with an increase in the infiltration of CD8+ T memory lymphocytes in the tumour microenvironment.
What are the consequences in practice?
Despite its limitations, this study suggested that microbiota modulation via FMT could increase ICI efficacy when administered in a first-line setting for metastatic melanoma. Although the wide-scale use of FMT seems difficult in current practice, modulating the microbiota, in particular with new-generation probiotics, in combination with ICI could become a standard treatment.