Key take aways on diarrhea
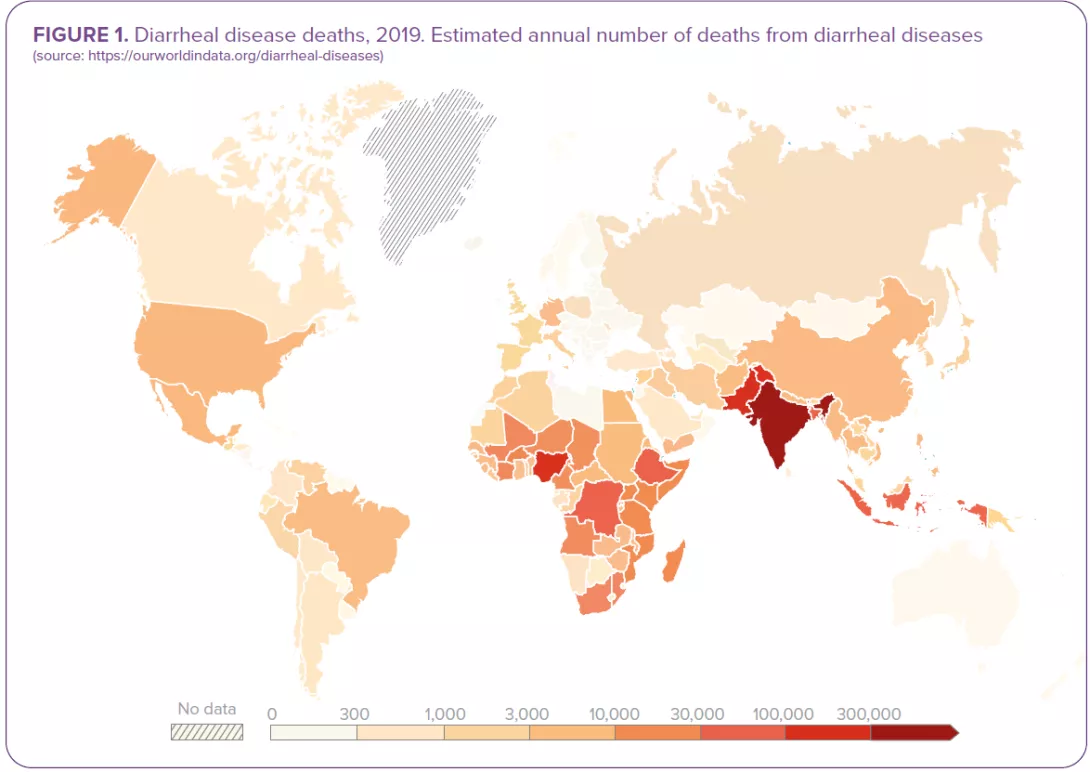
- Diarrhea kills around 1.5 million people every year.55 It is the third cause of death in children under 5.1
- Most cases of acute diarrhea are due to infectious pathogens, i.e. viruses, bacteria, parasites. Rotavirus and Escherichia coli are the two most common etiological agents of moderate-to-severe diarrhea in low-income countries.1
- Whatever the etiological agent of infectious diarrhea, the outcome depends on complex interplays between the pathogen and the gut microbiota.
- The composition of the gut microbiota can shape the outcome of an infection by a diarrheal pathogen, and be either a protective or a facilitating factor. In turn, the diversity and composition of gut microbiota can be severely altered by infectious diarrhea and a return to a "healthy microbiota" may require several weeks after diarrhea has resolved.14
- A significant proportion of diarrheal disease can be prevented through safe drinkingwater and adequate sanitation and hygiene.1
- Rotavirus vaccination is another important preventive strategy, which the WHO recommends be included in all national immunization programmes and considered as a priority.56
- The majority of infectious diarrheas are self-limiting in immunocompetent individuals. Nevertheless, some patients (with severe dehydration, more severe illness, persistent fever, bloody stools, immunosuppression…) require specific diagnostic investigation.11
- The most important complication of infectious diarrhea is dehydration, which may require oral or intravenous fluid replacement therapy, depending on the degree of dehydration.1
Both the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and the World Gastroenterology Organization (WGO) consider that some probiotic strains can be recommended by HCP:
- for the prevention of antibiotic-associated diarrhea;
- for the treatment of acute (viral) diarrhea in children, as they may shorten diarrhea duration.
- Future research should expand microbiome knowledge in the context of infectious diarrheas, in order to improve their prevention and management.
- The optimization of microbiota profile in order to shape infectious outcomes5 and improve rotavirus vaccine efficacy29 represents a promising research path.
Parasitic diarrhea: can the microbiota shape clinical outcomes ?
Not all individuals respond to intestinal infections of parasites in the same way: while some develop no symptoms at all, others experience more or less severe diarrhea, which can lead to death. The gut microbiota is increasingly cited as a key factor in explaining this variability
Intestinal parasites can be broadly classified into protozoa (single cell organisms) and helminths (multicellular, known as worms).39 Globally, there are an estimated 895 million people infected with soil transmitted helminths (STH). Intestinal protozoa (IP) have a lower overall prevalence rate, but still, over 350 million people are believed to be infected with 3 of the most common protozoan parasites40. Protozoan infections are common in low and middle income countries (LMICs). Food-chain globalisation, international travel and migration are leading to an increase in protozoan infections in high income countries, where they are more common than intestinal helminth infections.39
Giardiasis, the most common parasitic diarrhea worldwide, affects 280 million people each year. 41
DIARRHEAS CAUSED BY PROTOZOAN PARASITES
The most common intestinal protozoan parasites are Giardia intestinalis (Giardia duodenalis or Giardia lamblia), Entamoeba histolytica, Cyclospora cayetanensis, and Cryptosporidium spp. Diarrheal diseases caused by these pathogens are known respectively as giardiasis, amebiasis, cyclosporiasis and cryptosporidiosis.41
Giardia intestinalis infects the upper small intestine altering its barrier and permeability. Between 6 and 15 days after infection, it can cause acute, watery diarrhea associated with abdominal cramps, bloating, nausea and vomiting. Giardiasis, the most common parasitic diarrhea worldwide, affects 280 million people annually. 41
Entamoeba histolytica infections are usually asymptomatic but can produce an invasive disease of the large bowel (notably in immunocompromised patients) and amebic dysentery can develop. The acute phase lasts 3 weeks, with abdominal pain, bloody diarrhea and mucus in the stools. Accounting for over 26 000 deaths annually,2 amebiasis is the third leading cause of death from parasitic infections worldwide; it particularly affects people in LMICs.41
Cyclospora cayetanensis is the only species of the genus Cyclospora that can infect humans. After an incubation period which may vary from 2 to 12 days, it typically manifests as voluminous watery acute diarrhea, abdominal cramps, nausea, low grade fever, fatigue and weight loss.41
Cryptosporidium spp. infection symptoms appear after one or two weeks of incubation: the most common clinical symptoms are acute watery diarrhea, abdominal cramps, malabsorption, nausea, vomiting and fever, lasting for approximately 5 to 10 days.41 An estimated 64 million cases of cryptosporidiosis are reported each year.40
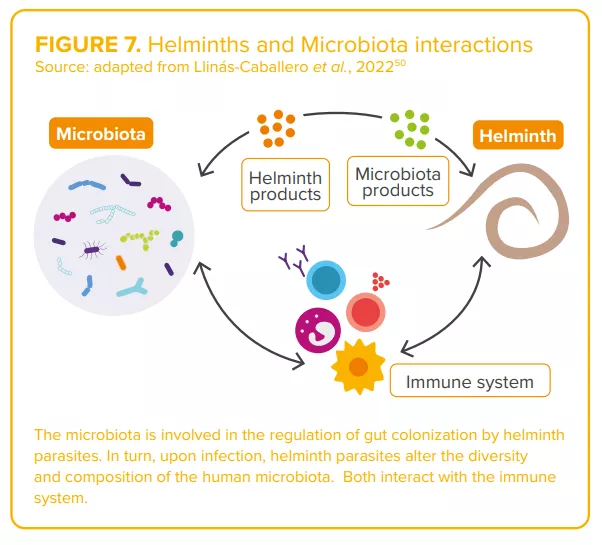
Helminth parasites and the microbiota have coexisted within their hosts, for millions of years. 50
TRAVELERS’ DIARRHEA: PARASITIC INFECTION IS COMMONLY ASSOCIATED WITH PI-IBS
While the majority of travelers’ diarrhea cases are acute and resolve spontaneously, a subset of individuals will experience persistent gastrointestinal (GI) symptoms potentially extending for weeks, months, or even years, after the initial cause has been effectively treated.52 A recent publication suggests that nearly 10% of patients experiencing travelers’ diarrhea develop persistent symptoms consistent with post-infectious irritable bowel syndrome (PIIBS). Parasitic infection, particularly giardiasis, is commonly associated with PI-IBS.53
DIARRHEAS CAUSED BY SOIL-TRANSMITTED HELMINTHS
Globally, the principal soil-transmitted helminths are the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura) and hookworms (Necator americanus and Ancylostoma duodenale). Symptoms experienced following helminth infection are related to the number of worms harboured: people with infections of light intensity (few worms) do not usually experience discomfort, whereas heavier infections can cause a range of symptoms including some that manifest in the intestine (diarrhea and abdominal pain), malnutrition, general malaise and weakness, and impaired growth and physical development. Soiltransmitted helminths contribute to the burden of diseases by impairing the nutritional status of the people they infect in a variety of ways: they feed on host tissues, cause intestinal blood loss and hamper the absorption of nutrients.42
- Ascaris lumbricoides is the most common intestinal nematode that infects humans, with an estimated 807 - 1,221 million people infected each year. 43 Infection commonly occurs without symptoms. The symptomatic form is characterized by an early lung phase followed by a later intestinal phase, which is characterized by diarrhea, mild abdominal pain, anorexia, nausea and vomiting.41
- An estimated 604-795 million people in the world are infected with Trichuris trichiura. People with heavy infections can experience frequent painful bowel movements that contain a mixture of mucus, water and blood.44
- An estimated 576-740 million people in the world are infected with hookworms, usually without symptoms. Few people, especially those infected for the first time, experience gastrointestinal symptoms. The most common and serious effects of hookworm infection are intestinal blood loss leading to anaemia, in addition to protein loss.45
MICROBIOTA: A ROLE IN THE MARKED CLINICAL VARIABILITY OF PARASITIC DIARRHEA?
Parasitic protozoan infections are characterized by marked variability in their clinical presentation: they can be asymptomatic or cause diarrhea, abdominal pain, weight loss, etc. Recent studies have highlighted the potential contribution of the intestinal microbiota to this clinical variation: for instance, an abundance of Prevotella copri in the gut microbiota predicted diarrhea in the context of Entamoeba histolytica infection;46 low Megasphaera abundance prior to and at the time of Cryptosporidium detection was associated with parasitic diarrhea in infants in Bangladesh, suggesting that the gut microbiota may play a role in determining the severity of a Cryptosporidium infection.47 In turn, infection by protozoan parasites alters the gut microbiome.48,49
Regarding helminths, the complex interactions between worms and the microbiota (“two of humans’ old friends” 50) are currently under study50 (Figure7). Authors agree on the existence of a complex and dynamic interplay between parasite(s), the host microbiota and host immunity, capable of shaping the clinical outcomes of parasitic infections. 46,48
CLINICAL CASE by Pr. Stephen Allen
- During her holiday in Asia, 36-year-old company executive develops non-bloody, slimy, smelly diarrhea with abdominal cramps and bloating.
- In the second week of the illness, stool microscopy revealed giardiasis and she takes a 10-day course of metronidazole.
- Over the course of the following year in the UK she experiences frequent episodes of similar symptoms, each lasting for a few days and forcing her to stay off work.
- After other illnesses are ruled-out by further investigations and a clinical review, she is diagnosed with post-infectious, diarrheapredominant, irritable bowel syndrome (IBS-D), a condition that develops in 10% of patients following an acute episode of gastroenteritis.54
- She finds that dietary changes and treatments for IBS-D have little effect and wants to know if she should send a stool sample abroad for microbiome analysis and whether a fecal transplant might help.
- The role of persistent dysbiosis in postinfectious IBS due to parasitic infection and/or drugs used for treatment is poorly understood. More research is needed before this woman’s questions can be answered with any confidence.

EXPERT OPINION
Gut parasite infection is a common cause of disease worldwide, predominantly diarrhea with protozoans such as giardia, Entamoeba histolytica and Cryptosporidium, and anaemia with helminths. Equally, gut parasites occur as commensals and may even bring health benefits such as improving resistance to other enteropathogens and preventing allergic and auto-immune diseases. The challenge is to gain a better understanding of the complex interrelationships between different parasites, the intestinal mucosa, gut immune cells and the gut microbiota in order to be able to exploit the benefits while at the same time ameliorating adverse effects of intestinal parasitic infection.
Viral diarrhea: will vaccines change the game ?
Usually presenting as watery diarrhea, viral diarrheas are caused by 5 main types of virus. Among them, rotavirus remains the leading cause of diarrhea-related mortalities in children under 5 years of age, this despite the availability of vaccines since 2006. Composition of the gut microbiota, implicated in viral infection outcomes, and rotavirus vaccine efficacy, could play a key role in strategies aimed at reducing the burden of viral diarrhea.
Rotaviruses, norovirus, sapovirus, astrovirus, and adenovirus: five virus types are currently recognized as the main causes of viral diarrhea.21
Of the 2 billion plus episodes of diarrheal disease that occur across the world each year, as estimated by the 2016 Global Burden of Disease (GBD) Study,2 almost 900 millions of the moderate-to-severe episodes were attributed to just three of these viruses: rotavirus, norovirus and adenovirus22.
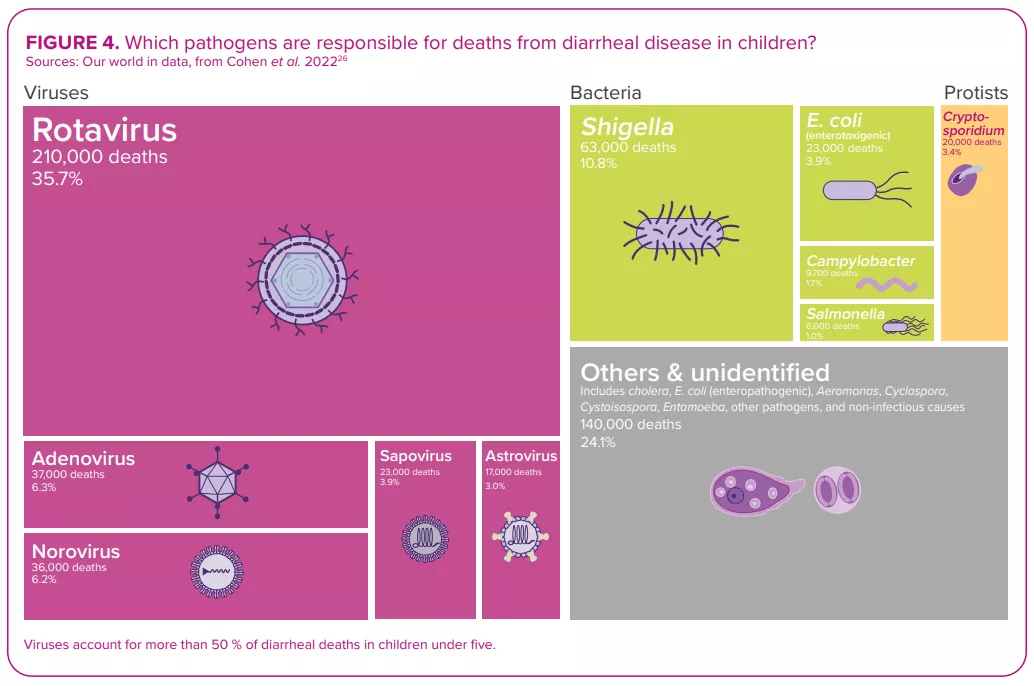
ROTAVIRUS, THE NUMBER ONE DIARRHEAL KILLER IN CHILDREN
Despite the development and availability of rotavirus vaccines since 2006,22 this virus, which causes more severe symptoms than most other enteric pathogens,22 was still responsible for over 228,000 deaths worldwide in 2016, of which over 128,000 occurred in children below the age of 52 – making rotavirus the leading cause of diarrhearelated mortalities among this segment of the population (Figure 4).
WATERY DIARRHEA
Whatever the virus that triggers an episode of diarrhea, the process of infection is broadly the same: the virus infects the epithelial cells of the small intestine and causes damage that hampers the absorption of fluid.21 Viral diarrhea usually manifests itself in the form of a watery (non-bloody) diarrhea. It can be accompanied by other symptoms, i.e. nausea, abdominal cramps, vomiting and fever,22 resulting in what is known as viral gastroenteritis.
REHYDRATION… AND PROBIOTICS
Just as for the other etiologies (bacterial or parasitic) of infectious diarrhea, management of viral diarrhea relies on oral or intravenous fluid replacement therapy, depending on the degree of dehydration.21 In addition, according to the latest conclusions of the ESPGHAN committee (2023),20 healthcare professionals may recommend some probiotic strains (L. rhamnosus, S. boulardii and L. reuteri) for the management of acute gastroenteritis in children, since there is some evidence (certainty of evidence: low; grade of recommendation: weak) of reduced duration of diarrhea, and/or length of hospitalization, and/or and stool output.
Among all diarrheal pathogens, and despite vaccine availability, rotavirus remains the number one killer of children under five years of age. 2
IMPROVING ROTAVIRUS VACCINE EFFICACY, A CHALLENGE YET TO OVERCOME
Regarding prevention, the usual preventive measures apply (ensuring safe drinking water, adequate sanitation and frequent handwashing, limiting contact with infected people, etc.). Given the considerable burden of rotavirus diarrheal disease, rotavirus vaccines are another important preventive measure. 22,23
SARS-COV-2: NEW MEMBER OF THE DIARRHEAL VIRUSES CLUB
Alongside the viruses long recognized as principal causes of viral diarrhea, infection with the SARS-CoV-2, responsible for the greatest pandemic in recent times, COVID-19, may also give rise to diarrhea. In clinical studies, the incidence rate of diarrhea ranges from 2% to 50% of cases. 27 As with the respiratory tract, the angiotensin-converting enzyme 2 (ACE2) receptors are highly expressed on intestinal cells, serving as an important site of entry for the virus in the gut. The putative mechanisms leading to the development of diarrhea mainly involve angiotensin-converting enzyme 2 dysregulations following virus entry into enterocyte, which could trigger an inflammatory response, ionic imbalance and increased permeability. Furthermore, the spike protein of SARS-CoV-2 acts as an enterotoxin, with a mechanism similar to the rotavirus enterotoxin NSP4.28 Alteration of gut microbiota and side effects of medications (antiviral and antibiotics) are also thought to be involved.29
Microbiota: a key role in rotavirus vaccination efficacy
Since their introduction in 2006, oral rotavirus vaccines (ORVVs) have at a global level led to a significant drop in the number of hospitalisations and deaths due to rotavirus diarrhea.30 However, the efficacy of the vaccines has been varied, with low-income countries suffering from lower performance compared to the remarkably high efficacy (>90%) observed in higher income countries.31 Reasons for this disparity are thought to be multi-factorial (host immunity, perinatal outcomes, genetics, nutritional status, stress, tobacco and alcohol use, rural versus urban location, family size, etc.). Just as for other vaccines, the composition and function of the gut microbiota is considered to be a key factor that regulates the immune response to vaccination30,32,33 (Figure 5).
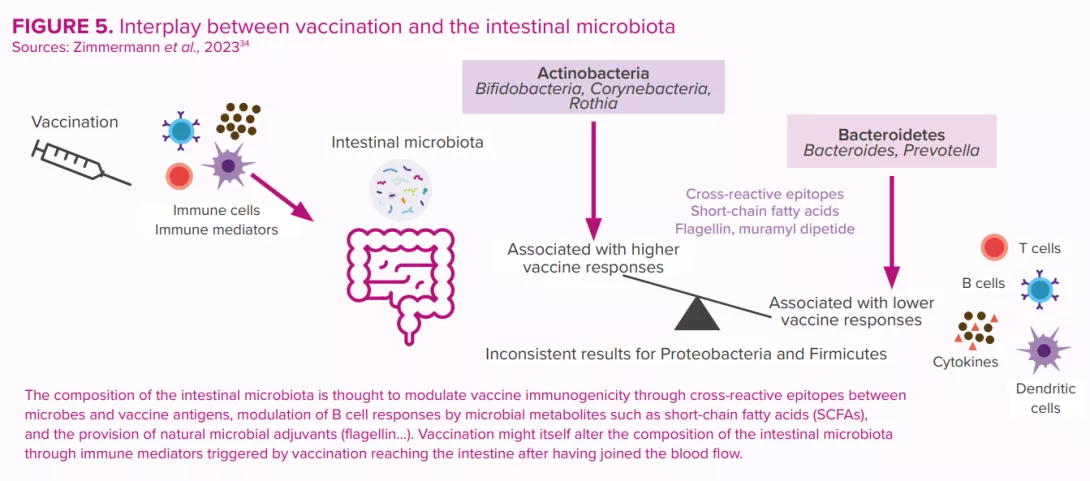
These were estimated to have prevented 139,000 deaths from rotavirus among under-fives during the period 2006 to 2019, and to have prevented 15% of under-five rotavirus deaths in 2019.24 However, vaccine efficacy is region-specific and displays poor seroconversion in low-and middle-income countries. Human clinical trial data have suggested a possible link between the gut microbiota and the enteric immune system’s response to rotavirus vaccine25 (Figure 5).
Each gram of human gut content is estimated to contain at least, 108 –109 virus-like particles, the vast majority of which are phages.14
MICROBIOTA: FRIEND OR FOE IN THE ONSET OF VIRAL DIARRHEA?
In cases of viral diarrhea, as with infectious diarrhea in general, the outcome of the confrontation between pathogen and host depends on complex balances that in large part involve the microbiota.
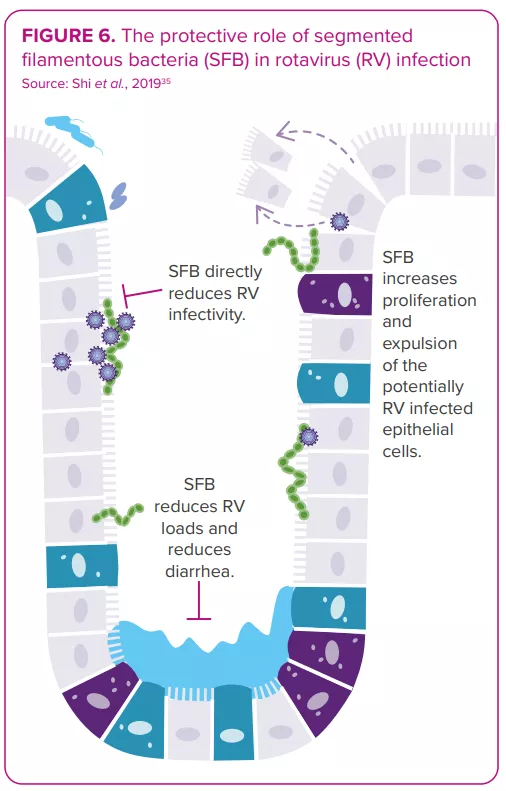
The gut microbiota displays bidirectional interactions with rotavirus and norovirus infections:14 it can either protect against or predispose the host to infection; in turn, an infection can alter the gut microbiota. Some bacteria seem able to inhibit viral infection. For example, one study shows segmented filamentous bacteria preventing and curing rotavirus infection in mouse colonies35 (Figure 6). On the other hand, in vitro and in vivo studies indicate the gut microbiota’s involvement in facilitating viral infection: certain gut microbes (e.g. Enterobacter cloacae) stimulate the ability of human norovirus to infect human B cells in vitro; microbiota elimination by antibiotics delays infection, reduces infectivity and/or viral titer of norovirus and rotavirus in mice.8,36
Therefore, any invasive pathogens might have different effects depending on the state of the gut microbiota. 3 The optimal microbiota profile and microbiota-targeting strategies that would lower the risk of infection and the following viral diarrhea remain to be characterized.37
As to the effect of viral infection on gut microbiota composition, numerous studies have documented specific patterns of dysbiosis in patients suffering from viral diarrhea compared to healthy controls25,38. A reduction of microbiota (alpha) diversity is often reported, but specific taxa increases or decreases vary widely across the studies.14 And one question remains: does the dysbiosis observed during viral diarrhea reflect a prior disposition that might have facilitated the infection, is it a state caused by the virus, or is it a combination of both?
CLINICAL CASE by Dr. Marco Poeta
- A 4-year-old girl presented to the paediatric emergency room with fever, diarrhea, vomiting and severe dehydration.
- Since the child needed intravenous rehydration, she was admitted to the hospital.
- The nasopharyngeal swab returned positive for SARS-CoV-2 infection, despite the absence of respiratory symptoms.
- Stools were negative for rotavirus, norovirus, adenovirus, bacteria and parasites but positive for SARS-CoV-2.
- Following the administration of probiotics, her stool frequency and consistency both recovered.
- The intravenous hydration was discontinued after four days, and the child was discharged.
- Diarrhea can be the only clinical manifestation of the SARS-CoV-2 infection. SARS-CoV-2 should therefore be added to the list of enteric pathogens.
- The efficacy of probiotics against Covidassociated gastroenteritis observed in this clinical case has already been demonstrated via in vitro studies.

EXPERT OPINION
Probiotics are recommended as an active means of treating of viral diarrhea in children, exerting an antidiarrheal effect that restores microbiota composition from its altered state. In clinical trials, some probiotic strains reduce secretory diarrhea in a very short time, measurable within hours after the start of probiotic administration. Considering that several days are normally required to establish changes in the composition of the microbiota, the rapid efficacy of probiotics implies that there are additional positive effects. Molecules secreted by the bacteria that act directly on intestinal cells can inhibit secretory diarrhea through an antioxidant mechanism. This is defined as the "postbiotic effect". The metabolites produced by probiotics have pharmacological-like action and could represent innovative therapies for the management of viral diarrhea.
Focus on antibiotic associated diarrhea (AAD)
Antibiotics are a powerful tool in the fight against bacterial infections, however they also disrupt the protective intestinal microbiota and this can lead to unintended consequences including antibiotic-associated diarrhea (AAD) in as much as 35% of patients.17,18,19 The incidence of AAD depends on several factors: 17,18,19 age (among children this percentage can reach up to 80%) 15, setting, type of antibiotic, etc. Most of the time, AAD is caused by antibiotic-induced dysbiosis, is of mild intensity and is self-limiting, lasting between 1 and 5 days.
While the etiologies for AAD are diverse, approximately one-third of AAD cases are attributed to C. difficile. Under certain conditions, C. difficile will trigger an inflammatory response leading to a range of clinical outlooks, from mild diarrhea to pseudomembranous colitis, toxic megacolon and/or death.17
ESPGHAN 2023 RECOMMENDATIONS
In 2023, the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Special Interest Group on Gut Microbiota and Modifications set out updated recommendations for the use of probiotics in the management of selected paediatric gastrointestinal disorders:20
“If the use of probiotics for preventing antibioticassociated diarrhea (AAD) is considered because of the existence of risk factors such as class of antibiotic(s), duration of antibiotic treatment, age, need for hospitalization, comorbidities, or previous episodes of AAD, healthcare professionals may recommend high doses (≥5 billion CFU/day) of S. boulardii or L. rhamnosus GG started simultaneously with antibiotic treatment to prevent AAD in outpatients and hospitalized children (certainty of evidence: moderate; grade of recommendation: strong).”
CLINICAL CASE by Pr. Aldo Maruy
- A 10-year-old patient came to the clinic with a seven-day history of diarrhea. From the onset, the child had been producing each day two or three liquid stools with mucus, though without blood. The mother said there had been neither fever nor vomiting. On clinical examination, the child seemed well and appeared to be adequately hydrated.
- The doctor requested a stool culture and OVA & parasite checks; these were negative.
- An antecedent had not initially been considered: six weeks before, the child had had a respiratory infection which was treated with antibiotics.
- Late-onset Antibiotic Associated Diarrhea (AAD) was then suspected. The patient received probiotics and improved within a week.
- AAD can take anywhere from 2 hours to 8-10 weeks to develop after antibiotic use.

EXPERT OPINION
Antibiotic Associated Diarrhea (AAD) is a common side effect of antibiotics. Age, spectrum of antibiotics used, underlying illness and recent surgery have been identified as risk factors. Recent evidence shows a new one: composition of the microbiota. In patients treated with β-lactams, higher relative abundances of Bacteroides were inversely associated with AAD while higher baseline abundance of Bifidobacterium species and Lachnospiraceae and amino acid biosynthesis pathways (AABP) were associated with AAD. Relative abundances of potentially protective taxa and levels AABP may distinguish children who did and did not experience AAD. Further studies are needed to investigate whether similar trends are observed across different antibiotic types. The identified potentially protective taxa may inform the development of preventive approaches for AAD.
Bacterial diarrhea: gut microbiota, a potential victim or a guard against it?
Pathogenic bacteria such as Shigella, Vibrio cholerae, Salmonella, E. coli… lead to bacterial diarrhea, through mechanisms that depend on the bacteria involved. Bacterial diarrheas are accompanied by intestinal dysbiosis. Symmetrically, the gut microbiota exerts effects on the bacterial infection. Since a ‘healthy’ gut microbiota is more resistant to infection, probiotics could reduce the severity of many bacterial infections.
Underlying how lethal bacterial diarrhea can be, these 8 bacteria were responsible for more than a third of the over 1.65 million deaths from infectious diarrhea recorded worldwide in 2016.2
These 8 bacteria
- Shigella: 212,438 deaths.
- Vibrio cholerae: 107,290 deaths.
- Non-typhoidal Salmonella spp: 84,799 deaths.
- Campylobacter spp: 75,135 deaths.
- Enterotoxigenic E. coli: 51,186 deaths.
- Clostridioides difficile: 22,417 deaths.
- Aeromonas: 16,881 deaths.
- Enteropathogenic Escherichia coli: 12,337 deaths.
FROM INFECTION TO DIARRHEA
The mechanisms that lead to bacterial diarrhea depend on the bacteria involved. Transmitted via contaminated food, water or by personto-person contact, Shigella infests the gastrointestinal tract, produces an enterotoxin and serotype toxin 1, destroying the intestinal epithelium and leading to severe bloody and mucous diarrhea.3,5
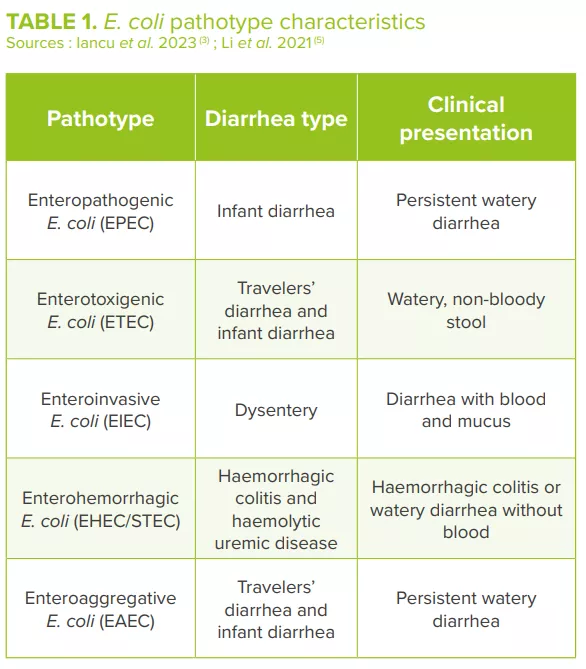
Pathogenic variants of Vibrio cholerae produce a cholera toxin that activates anion secretion, inhibits absorption of electroneutral NaCl and destroys the intestinal barrier function, thereby inducing massive fluid secretion in the lumen of the small intestine and the loss of large amounts of water, sodium, chloride, bicarbonate and potassium.3,5,13 Different pathogenic E. coli strains, classified into different pathotypes (Table 1), cause mild to severe diarrhea usually accompanied by fever. E. coli adheres to the intestinal epithelial cells through the adherent fimbriae, produces toxins and exerts its pathogenic effects.3,5
EFFECT OF PATHOGENS AND DIARRHEA ON MICROBIOTA
Bacterial diarrheas are accompanied by dysbiosis, usually with an overabundance of facultative anaerobes (Escherichia, Streptococcus, Enterococcus, etc.) in dysenteric diarrhea, and a depletion in bacteria of known immuno-modulatory effects (Lactobacillus ruminis, Bifidobacterium pseudocatenulatum)7 (Figure 3).
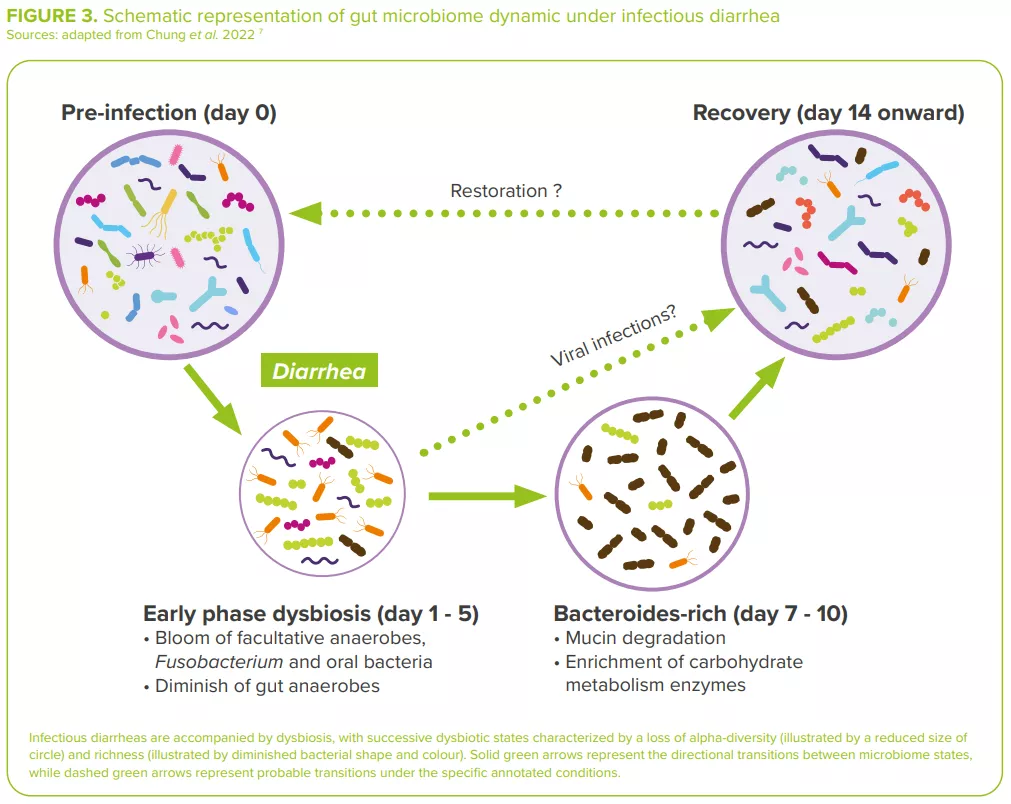
For example, in cholera, the gut microbiota is substantially modified both during and after infection, this as a consequence of the removal of the mucus layer along with the residing gut microbial community and of the delivery of toxin by V. cholerae. 13 During recovery, the gut microbiota of cholera patients slowly repopulates via an accumulation pattern similar to that of the maturation of the gut microbiota observed in children.3
In the same way, children infected with diarrheagenic E. coli (DEC) display a distinctive gut microbial composition, with a high fraction of Bacteroidetes and Proteobacteria and a reduced abundance of Firmicutes. 13 Increases in Proteobacteria could be partially explained by an increase in Escherichia/ Shigella species (as the cause of diarrhea) and in other members of Enterobacteriaceae, such as Citrobacter and Enterobacter (related to histamine production induced by proinflammatory environments and associated with E. coli adherence).14 Frequent antimicrobial use can also partly explain the observed dysbiosis.7
GUT MICROBIOTA PROTECTION AGAINST INFECTIONS
Symmetrically, the gut microbiota has demonstrated effects on bacterial infection. In germ-free animals, their lack of a gut microbiota and the absence of ecological competition results in an immature immune system that renders them highly susceptible to diarrheal pathogens: 10 colony-forming units (CFU) of Salmonella are enough to cause a lethal infection, whereas to kill 50% of mice with an intact gut microbiota 103 –109 CFU are required.8
In humans, Prevotella, Bifidobacterium and Blautia have been shown to reduce colonization of V. cholerae. Conversely, Paracoccus is believed to support the growth of the pathogen.13 This is the reason why promoting a ‘healthy’ gut microbiome has been considered a useful approach in the intervention and prevention of cholera.13
“The GI tract encompasses ~1 to 10 times more bacterial cells than the number of cells in the human body.” 16
BACTERIAL AND YEAST PROBIOTICS, PREBIOTICS AND FMT
The severity of several bacterial infections could be reduced by probiotics: for example, probiotic E. coli inhibits the biofilm formation of other E. coli strains and likewise those of the pathogenic Staphylococcus aureus and S. epidermidis. 3 Regarding dysentery, a combination of Lactobacillus and Bifidobacterium strains and a Streptococcus strain reduces both the duration of blood-affected diarrhea and the time spent in hospital.3
Numerous mechanisms could explain why probiotics alleviate diarrhea:3 production of antimicrobial substances, competitive exclusion, competition with cell binding sites, production of acids and metabolites that lower the surrounding pH, strengthening of the gut mucosal barrier, modulation of gut mucosal immunity and gut microbiota diversity. For example, the probiotic yeast Saccharomyces boulardii may facilitate gut microbiota restoration in children with acute diarrhea.15
Prebiotics can also have a positive impact on diarrhea: by increasing the bacterial production of short-chain fatty acids (SCFAs) such as butyrate, which contribute to the gut barrier integrity; and by antagonizing the adherence of pathogens to epithelial cells, thus inhibiting colonization and promoting gut pathogen elimination.3
Fecal microbiota transplantation (FMT), which aims to restore a healthy gut microbiota, has proven effective and is only indicated in the treatment of recurrent C. difficile in both adults and children.14
CLINICAL CASE by Pr. Aldo Maruy
- A 2-year-old boy presented with fever, abdominal pain and diarrhea with mucus and blood. He had history of two similar episodes in the last six months, treated solely with antibiotics.
- In order to prevent a relapse, it was decided to treat him with antibiotics and probiotics. The diarrhea ceased within 48 hours, the antibiotic was suspended at the 5th day while the probiotic was continued for two weeks; a diet rich in complementary food and prebiotics was prescribed.
- In addition to treating the infection with antibiotics, it is recommended in order to prevent a new diarrheal episode that the composition of the gut microbiota be restored via the diet together with the administration of prebiotics and probiotics

EXPERT OPINION
Throughout one’s life, a healthy microbiota plays an important role in the prevention and treatment of bacterial diarrhea. Specific species have demonstrated protective effects against diarrhea: Lactobacillus taxa are protective against Shigella spp.-induced diarrhea; the presence of Sutterella sp., Prevotella copri and Bacteroides vulgatus predict resistance to enterotoxigenic E. coli (ETEC). On the other hand, microbial intervention, through modifying the diet and using prebiotics, probiotics and FMT, can regulate the composition of gut microbiota to prevent and treat diarrhea. Future research should expand our knowledge of the microbiome as it relates to infectious diarrhea, thereby assisting in the design of improved preventative and managerial interventions.
CONSEQUENCES OF TRAVELERS’ DIARRHEA
When visiting medium- and high-risk destinations, 10–70% of travelers from low infectious disease-risk countries experience diarrhea. Travelers’ diarrhea is predominately caused by bacteria (≥80%–90% of cases), with intestinal viruses accounting for a minimum of 5%–15% of the cases.52 Infections by protozoal pathogens might account for approximately 10% of diagnoses mostly in those traveling for extended periods.
The microbiota of travelers who experienced diarrhea show greater variation throughout the length of their stay than those of healthy travelers, associated with a lower baseline diversity, which has been linked to an increased susceptibility to infection.51
Moreover, diarrhea reduces the capacity of microbiota restoration (large increase of the rate of divergence from baseline) and leads to the acquisition of multidrugresistant organisms.51 Thus, according to a study including 267 Americans travelling outside the United States, a third returned with diarrhea, 61% with intestinal dysbiosis and 38% with antibiotic-resistant bacteria (most of them an E. coli), contributing to the global spread of antimicrobial resistance.58
E. coli, Dorea fomigenerans, Bacteroïdes vulgarus, B. caocae, Odoribacter splanchnicus…,
Ruminococcus bromii, coprococcus, Clostridioides bartlett
Microbiota and Infection Diarrhea: virtuous or vicious circle ?

INFECTIOUS DIARRHEA, ONE OF THE LEADING CAUSES OF INFANT MORTALITY WORLDWIDE
The passage of 3 or more loose or liquid stools per day corresponds to the common definition of diarrhea as set out by the WHO.1 Both criteria (frequency and consistency) are necessary: frequent passing of formed stools is not diarrhea, nor is the passing of loose stools by breastfed babies (Figure 1). 1.6 million deaths were attributed to diarrhea in 2016.2 Children are particularly at risk: diarrheal disease is the 3rd leading cause of death in children under 5 years of age. A large part of the mortality used to be attributed to severe dehydration linked to fluid loss but today, septic bacterial infections account for an increasing proportion of all diarrhea-associated deaths.1 Malnourished or immunity-impaired children are the most at risk of life-threatening diarrhea, as well as people living with HIV.1
DIARRHEA CLASSIFICATION
There are 3 clinical types of diarrhea based on its symptoms and duration:1
- acute watery diarrhea, which lasts several hours or days (up to 14 days) and includes cholera;
- acute bloody diarrhea (dysentery);
- persistent diarrhea, which lasts 14 days or longer.
Most cases of acute diarrhea are due to infections:1,3,4 a virus, bacteria or parasites could all be responsible, but rotavirus and Escherichia coli are the two most common etiological agents of moderate-to-severe diarrhea in low-income countries.1 Rotavirus and Shigella are responsible for the highest number of infectious-diarrhea related deaths;2 this is giving rise to preventive vaccination strategies (still under development for the latter). Although certain fungal communities have been confirmed to be associated with diarrhea, the role of fungi in diarrhea remains controversial.5 They could be at work in some clinical settings, especially in immunocompromised patients prone to invasive fungal infections (candidiasis).6
PATHOPHYSIOLOGICAL SYNDROMES OF DIARRHEA
From the clinical perspective, diarrheagenic pathogens can cause 2 pathophysiological syndromes:4
- Noninflammatory diarrhea (NID): patients present with nausea, vomiting, watery and voluminous stools, and abdominal cramping resulting from intestinal secretion (the intestinal mucosa remains intact). This milder course of disease is usually viral (Rotavirus, Norovirus…) but it can also be bacterial (enterotoxigenic Escherichia coli, Clostridium perfringens…) or parasitic (Giardia, …);
- Inflammatory diarrhea (ID): patients present with fever, abdominal pain, tenesmus and bloody stools of smaller volume than in NID. This severe course of disease is usually caused by invasive or toxinproducing bacterial strains (Shigella, Salmonella species…) that lead to mucosal barrier disruption and tissue destruction.
“Rotavirus and Escherichia coli are the two most common etiological agents of moderate-to-severe diarrhea in low-income countries.”
BEHIND THE SCENES OF DIARRHEA: THE MICROBIOTA
VICIOUS CIRCLE: WHEN DIARRHEA LEADS TO GUT DYSBIOSIS
Infectious diarrhea is regarded as a major dysbiotic event resulting from:
- increased bowel movements and disruption to mucosal integrity, 3
- increased proportion of water in the fecal matter and reduced transit time which contributes to taxonomic scarcity, 3
- and possible oral rehydration, zinc supplements, probiotics and even antimicrobials (in the case of dysentery or bacterial infections) which also contribute to an imbalance in the intestinal microbiota.7
Depending of the type of infection, infectious diarrheas are usually accompanied by dysbiotic states: 7 bacteria-induced diarrhea is generally linked to an elevation in Escherichia, Streptococcus and oral bacteria; viral infections lead to a less pronounced reduction in anaerobic commensals in the gut (higher abundance in Bifidobacterium); giardia-induced diarrhea is linked to a decrease in Gammaproteobacteria and an enrichment in Prevotella.
“The community of microbes that inhabit the gut is as numerous as human cells, with the vast majority of bacteria residing in the colon.” 8
Dysbiosis corresponds to the disruption of a formerly stable, functionally complete microbiota.9
The community of microorganisms - bacteria, viruses, fungi (including yeasts) and parasites - that inhabit the gut.10
VIRTUOUS CIRCLE: WHEN THE GUT MICROBIOTA OFFERS PROTECTION
Mechanisms by which the gut microbiota provides colonization resistance can be both direct and indirect. The microbiota directly inhibits diarrheal pathogens mainly via competition for nutrients, but also by limiting in a variety of ways the growth of diarrheal pathogens: secreting bacteriocins (antimicrobial peptides), cell contact-dependent inhibitory structures (type VI secretion system), producing molecules that reduce the pathogens’ virulence…
The microbiota also indirectly inhibits diarrheal pathogens via its effects on the host: by promoting gut barrier maintenance and by stimulating both the innate and the adaptive immune system.8
HOW TO MANAGE INFECTIOUS DIARRHEA?
The majority of intestinal infections are self-limiting in immunocompetent individuals. Nevertheless, some patients (with severe dehydration, more severe illness, persistent fever, bloody stools, immunosuppression…) require specific diagnostic investigation. These can include a complete blood count, a creatinine and electrolytes assessment, verification of leukocytes and lactoferrin presence in the stools, stool culture, along with C. difficile testing, PCR, ova and parasites search, endoscopy and abdominal imaging11. The American College of Gastroenterology (ACG) guideline12 provides recommendations for the diagnosis and management of adult patients presenting with acute diarrhea of suspect infectious etiology (Figure 2). Clinical investigation in children is based on the same principles.23 In 2023, World Gastroenterology Organisation Global Guidelines included probiotics in the prevention and treatment of some infectious diarrhea57.
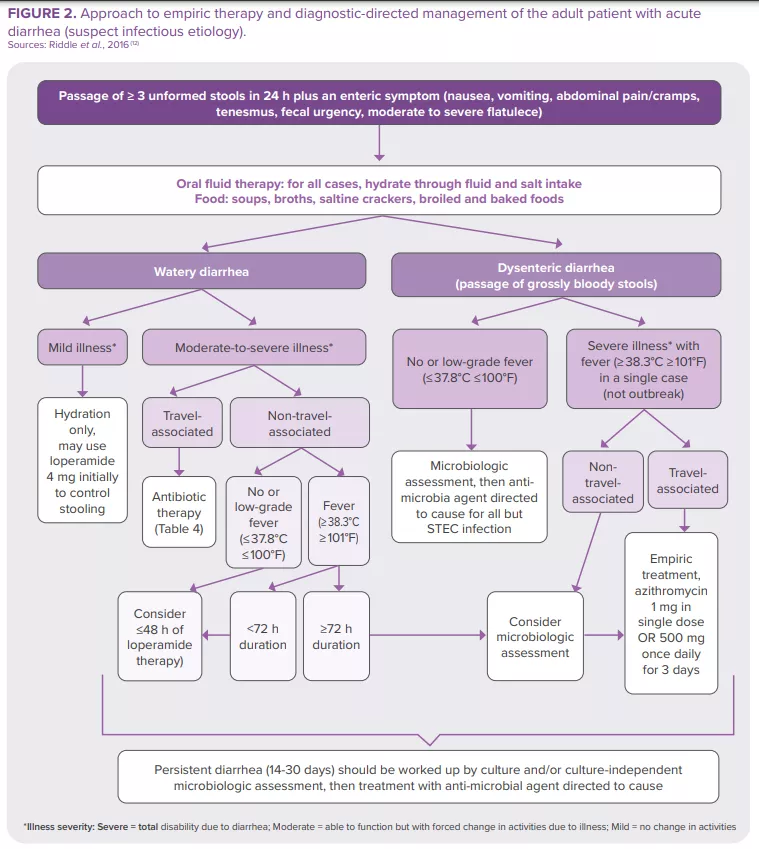
World Gastroenterology Organisation Global Guidelines, 2023
Treatment of acute diarrhea: “oral administration [of some probiotic strains] shortens the duration of acute diarrheal illness in children by approximately 1 day.”57
Prevention of:
- acute diarrhea: “probiotics probably make little or no difference with diarrhea lasting 48 hours or longer.”
- antibiotic-associated diarrhea: “probiotics may provide a moderate effect for preventing antibiotic-associated diarrhea in children, adults, and elderly adults.”
- Clostridioides difficile diarrhea: “probiotics are effective for preventing C. difficile–associated diarrhea in patients receiving antibiotics.”
From the mouth to the colon: tracing the route of Fusobacterium
A surprising new study reveals that Fusobacterium nucleatum, typically an oral bacterium, migrates to the colon and dominates colorectal cancer tumors. Researchers found that a distinct clade, Fna C2, not only thrives in these tumors but also actively promotes cancer growth.
New study has identified a specific clade of the bacterium (sidenote: Fusobacterium nucleatum Fusobacterium nucleatum is a Gram-negative, anaerobic bacterium commonly found in the human oral cavity. It plays a role in periodontal diseases and has been associated with various infections and inflammatory conditions. Fusobacterium nucleatum is notable for its ability to adhere to and invade host tissues, facilitating interactions with other microorganisms and host cells. ) (Fn) that dominates the (sidenote: Colorectal cancer Colorectal cancer is a type of cancer that begins in the colon (large intestine) or the rectum. It typically starts as small, benign clumps of cells called polyps that form on the inner lining of the colon or rectum. Over time, some of these polyps can become cancerous. ) (CRC) niche, revealing significant insights into the bacterium's role in cancer progression. 1 This groundbreaking study, led by Fred Hutchinson Cancer Center researchers, uncovers genetic and functional distinctions in Fn strains associated with CRC, highlighting potential targets for therapeutic interventions.
Identifying the culprit
The research team collected and sequenced genomes from 135 Fn strains, including 80 from healthy oral cavities and 55 from CRC tumors. Using advanced genomic techniques, they performed a pangenomic analysis, which identified 483 genetic factors enriched in CRC-associated strains. The findings revealed that the Fn subspecies animalis (Fna), previously considered a single subspecies, is actually composed of two distinct clades: Fna C1 and Fna C2. Among these, only Fna C2 was found to dominate the CRC tumor niche, suggesting a unique role in cancer development.
Fusobacterium nucleatum was detected in 29.2% of stool samples from patients with CRC and 4.8% of stool samples from healthy individuals.
Genetic and functional Insights
Further analysis showed that Fna C2 possesses 195 genetic factors associated with increased metabolic potential and gastrointestinal colonization, distinguishing it from Fna C1. This clade-specific genetic repertoire includes genes that enhance the bacterium's ability to invade host tissues and evade immune responses. The study also demonstrated that Fna C2-treated mice developed significantly more intestinal adenomas compared to those treated with Fna C1 or control groups. This suggests that Fna C2 not only colonizes CRC tumors but also actively promotes tumor growth.
Implications for treatment
These findings have profound implications for understanding the microbiome's role in CRC and developing targeted treatments. The study highlights the potential of focusing on Fna C2 for therapeutic interventions. For instance, identifying and targeting the specific metabolic pathways and virulence factors unique to Fna C2 could lead to new strategies for preventing or treating CRC. Additionally, the research underscores the importance of considering bacterial clades rather than species alone in microbiome studies, as significant differences can exist within subspecies.
The discovery of the distinct Fna C2 clade and its association with CRC marks a significant advancement in microbiome research and cancer biology. By delineating the genetic factors that enable Fna C2 to thrive in the CRC environment, this study opens new avenues for targeted therapies and improved patient outcomes.
As the role of the microbiome in cancer becomes increasingly evident, focusing on specific bacterial clades may prove crucial in developing effective treatments. This research sets the stage for further exploration into the intricate relationship between our microbiota and cancer, potentially transforming our approach to cancer therapy.
 Aspirin: antibiotic effect in colorectal cancer?
Aspirin: antibiotic effect in colorectal cancer?
 Colorectal cancer: a bacterium is a key player in chemoresistance
Colorectal cancer: a bacterium is a key player in chemoresistance
Gut dysbiosis in men: what are the consequences for their children?
Testicular physiology is strongly affected by gut dysbiosis. This could have unfortunate consequences for the health of future children. This is the main finding of a study carried out on mice.
Is there an axis of communication between the gut and germ cells? Yes, according to a new study published in Nature. 1
Dysbiosis, harmful to the reproductive system and future children
Researchers at the European Molecular Biology Laboratory in Heidelberg and Rome induced gut dysbiosis in male mice by administering either non-absorbable antibiotics (which do not cross the gastrointestinal epithelium) or osmotic laxatives. They then mated them with healthy females and analyzed their offspring.
Result: the pups suffered not only from lower birth weight and severe growth restriction, but also an early mortality rate 3.5 times higher than that of litters from healthy males.
Analysis of the reproductive systems of the fathers suffering from dysbiosis revealed various metabolic, physiological and hormonal changes:
- lower testicular weight
- changes in the structure of the seminiferous tubules
- different quantities of certain metabolites involved in germ cell functions
- deregulation of certain genes, notably a significant drop in the expression of the leptin gene, a hormone produced by adipocytes, but also by germ cells
Leptin, a key signaling factor?
Even more interestingly, in male mice mutant for the leptin gene (mimicking the effect of dysbiosis on leptin gene activity), the researchers noted characteristics similar to dysbiotic males (lower testicular weight and abnormal seminiferous tubules). Their sperm also showed changes in the abundance of certain "microRNAs" known to play a role in placental development during gestation.
By crossing these mutants with normal females, the researchers noted that the embryos showed a significant number of genes differentially expressed compared with embryos from healthy males. For the researchers, this demonstrates that leptin may well be an important signaling molecule in the gut-germline axis.
Microbiota influence on women's fertility
By interacting with estrogens, androgens and insulin, the gut microbiota plays a major role in female endocrine systems. Studies show that an imbalance can lead to complications during pregnancy, as well as diseases associated with infertility such as polycystic ovary syndrome or endometriosis. The gut microbiota is now considered an endocrine organ in its own right. 2
Severely disrupted placental development
Analysis of fetuses from mating with antibiotic-treated males and their placentas showed down-regulation of several important factors in placental development.
Among the main genes differentially expressed in placentas from crosses with antibiotic-treated males versus placentas from crosses with healthy males, the researchers found several clinical markers of placental insufficiency, such as those for pre-eclampsia.
Also present were:
- reduction of certain areas of the placenta (labyrinthine zone)
- altered placental vascularization
- a decrease in the placental growth factor
In a nutshell
For the first time, this study demonstrated that the environment can, via modulation of the gut microbiota, lead to sperm defects that can alter placental function and, therefore, the health of future children. It now remains to be seen whether a gut-germline axis also exists in humans... More to follow!
 Male infertility: is sperm microbiota involved?
Male infertility: is sperm microbiota involved?
 Endometrial microbiota: a new marker for IVF success?
Endometrial microbiota: a new marker for IVF success?
Disrupted paternal microbiota: unhealthy young
Male mice suffering from an imbalanced gut microbiota at the time of mating reportedly produce offspring with serious health problems. This is the surprising result of a study recently published in the prestigious journal Nature.
The gut microbiota
Should a man wishing to have children postpone conception if he has been prescribed antibiotics, which are likely to cause gut dysbiosis?
It is quite possible, according to a new study 1 that, for once, highlights men's responsibility for the future health of their offspring. Conducted on mice, this study shows that males suffering from dysbiosis at the time of mating father unhealthy offspring. Fortunately, when the microbiota is rebalanced, this transmission no longer occurs.
Transgenerational effects of gut dysbiosis
To reach this discovery, researchers at the European Molecular Biology Laboratory 2 in Heidelberg and Rome exposed male mice to 6 weeks of antibiotics. The aim was to induce (sidenote: Dysbiosis Generally defined as an alteration in the composition and function of the microbiota caused by a combination of environmental and individual-specific factors. Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. ) in the mice. They then mated them with healthy females, and the litters were analyzed after 3 weeks.
The result: compared to the offspring of fathers without dysbiosis (controls), mouse pups conceived by "dysbiotic" fathers:
- had a lower birth weight (a risk factor for diseases such as diabetes),
- suffered more from stunted growth,
- had a higher mortality rate in the first 2 months.
To explain these surprising results, the scientists analyzed the reproductive systems of the antibiotic-treated males.
A direct impact on placental development
They noted that their testes had reduced size and sperm count and showed significant hormonal and metabolic changes. The sperm showed differences in certain molecules involved in the development of the placenta.
These data prove that the microbiota and germlines (the cells that transform into sperm) communicate with each other, and that there is therefore a "gut-sperm axis."
- When analyzing the placentas of females mated with dysbiotic males during gestation, the researchers noted:
- poor placental vascularization, which could explain certain abnormalities in the offspring;
- the presence of markers similar to those of pre-eclampsia, a disease linked to abnormal placental development, responsible for intra-uterine growth restriction.
Fertility plummeting worldwide
Bad times for fertility... Between 1973 and 2018, the average sperm concentration in men fell from 101 to 49 million per milliliter of semen worldwide, a drop of almost 50% in 50 years! 3
The rate of decline is said to have doubled since the early 2000s, reaching 2.64% per year, and contrary to what one might think, not just northern countries are affected. South America and Africa are also reported to be experiencing a similar drop in fertility.
Exposure to substances such as bisphenols (A or S), phthalates, parabens and paracetamol are thought to be major causes of this deterioration. 4 Turbulent times ahead for the gut-sperm axis!
Towards a better pregnancy?
This is the first time that this kind of effect of the microbiota on the male reproductive system has been demonstrated in mammals.
Is there also a gut-sperm axis in humans? As yet, this has not been confirmed, and further studies will have to be carried out.
Considering that our lifestyles are likely to cause dysbiosis (poor diet, pollution, medications, etc.), proof of the existence of such an axis could pave the way for innovative approaches to ensure better reproductive health and more optimal pregnancies.
 Male infertility: are bacteria in semen involved?
Male infertility: are bacteria in semen involved?
 Everything you always wanted to know about penile microbiota (but were afraid to ask)
Everything you always wanted to know about penile microbiota (but were afraid to ask)