Longitudinal multi-omics analysis reveals subset-specific mechanisms underlying irritable bowel syndrome
Commented articles
By Prof. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France

Commentary on the original article by Mars et al. Cell 2020 [1]
The gut microbiome is implicated in numerous chronic gastro-int estinal disorders in humans. Determining its role however has been rendered difficult due to the lack of correlation between animal and human studies as well as that of an integrated multi-omics view of disease-specific physiological changes. The authors integrated longitudinal multi-omics data from the intestinal microbiome, the metabolome, the host epigenome and the transcriptome in the context of irritable bowel syndrome (IBS) host physiology. They have identified IBS sub-type specific and symptom-related variations in microbial composition and function. A sub-group of changes identified in microbial metabolites corre sponds to host physiological mechanisms which are relevant to IBS. By compiling multiple data layers, the authors identified purine metabolism to be a new host-micro biota metabolic pathway in IBS with potential for therapeutic application. This study highlights the value of longitudinal sampling and the integration of complementary multi-omics data in the identification of functional mechanisms which may be future therapeutic targets in a global treatment strategy for chronic intestinal diseases.
WHAT DO WE ALREADY KNOW ABOUT THIS SUBJECT?
Irritable bowel syndrome (IBS) is a disorder observed in patients all over the world, characterised by recurrent abdominal pain or discomfort. Occurring mainly in women, IBS is associated with changes in the form or frequency of stools and it is the form of the latter which defines the IBS subsets: IBS with constipation (IBS-C), IBS with diarrhoea (IBS-D) or IBS with mixed bowel habits (IBS-M).
The pathogenesis of IBS involves changes in gastro-intestinal motility, intestinal secretion, visceral hypersensitivity and intestinal permeability, all of which can be modified by the gut microbiome [2]. Moreover, IBS symptoms are influenced by the diet, host genetics and the environment, which are also known to modulate the human gut microbiome [2]. Experimental evidence supporting the role of the gut microbiome in IBS are based on patient-to-gnotobiotic mouse transplantation studies which reproduced certain symptoms associated with IBS-C and IBS-D (transit time, sensation of pain, intestinal permeability…) [3]. However, in the absence of robust animal models of IBS, studies in humans are needed to determine the interactions between the gut microbiome and the pathological pathways specific to humans. Human IBS studies are limited in general by the use of transversal sampling and a failure to stratify into patient subsets, which is reflected in the lack of agreement in the results obtained in the large number of studies on the microbiome [4].
The well-described influence of gastro-intestinal transit on the intestinal microbiome increases the variability of the studies even more. In addition, IBS, in common with other chronic gastro-intestinal disorders, is characterised by periods of remission and flare up of the symptoms, and transversal sampling therefore fails to take into account the fluctuations of the disease over time. Lastly, the inherent differences in host physiology between humans and animals have been an obstacle to progress in our understanding of the mechanistic roles of the gut microbiome in IBS. The authors performed a longitudinal study in subsets of patients with IBS, integrating multi-omics measurements, including the microbial metagenome, host transcriptome and the methylome with assessment of host cell functions. This revealed mechanisms specific to the IBS subset induced by impaired microbial metabolism, which corresponded to simultaneous changes in the host physiology.
Key point
-
The functions of the gut microbiota are impaired in IBS and there are differences between IBS-C and IBS-D
-
The increase in the production of tryptamine and the reduction in transformation of bile acids may be implicated in IBS-D
-
Over-consumption of hypoxanthine by the microbiota and host cells may be implicated in IBS by altering the energy level of the intestinal mucosal epithelium cells.
WHAT ARE THE MAIN INSIGHTS FROM THIS STUDY?
Here, the authors conducted a prospective observational longitudinal study with multi-omics analysis of the gut microbiome and the host cells. Healthy subjects were compared with patients with IBS-C and IBS-D. A total of 77 participants supplied at least one stool sample (a total of 474 stool samples were obtained), and 42 participants received a sigmoidoscopy which provided colon biopsies. In order to identify the microbial factors causing the symptoms specific to each of the IBS subsets, metagenomic sequencing and a metabolomic analysis were performed on the stool samples. A metabolomic analysis and cytokine assays were performed on serum samples. Lastly, 16S sequencing, and metabolomic, transcriptomic and methylomic analyses were performed on the colon biopsies. The authors identified differences in the composition and diversity of the gut microbiota between healthy subjects and IBS-C or IBS-D patients.
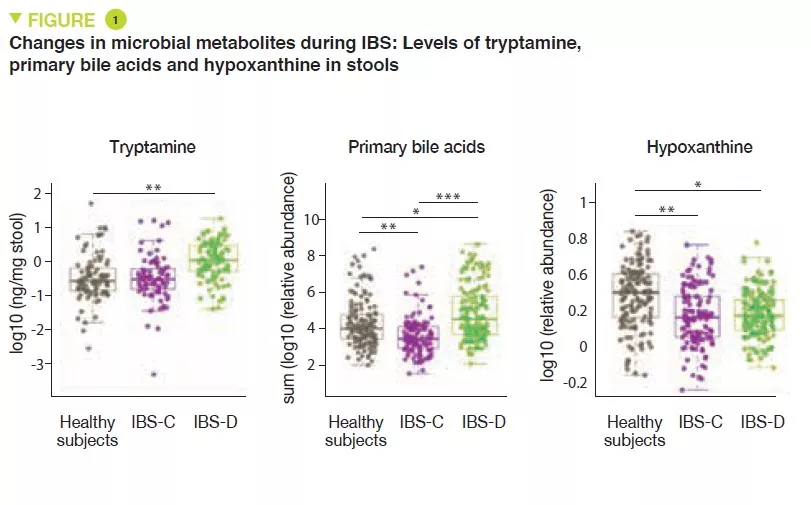
Metabolomic analysis of the stools revealed increased levels of tryptamine, a tryptophan metabolite produced by certain intestinal bacteria, in patients with IBS-D (Figure 1). Because tryptamine accelerates transit due to an action on the serotonin receptor, 5-HT4, it is suggested that it could have a role in the phenotype of these patients. Similarly, the proportion of primary bile acids was higher in patients with IBS-D, a sign of impaired transformation of bile acids by the microbiota. In vitro experiments suggest that primary bile acids increase secretion in the colon and could also participate in the phenotype.
Lastly, the integration of multi-omics data identified a potential new mechanism in IBS. The results suggest that in IBS patients there is increased degradation of purine nucleotides and of hypoxanthine in particular, by the microbiota and the host cells, which induces stress in the colon. It is suggested that this could lead to a compensatory response with an increase in purine recuperation. The low levels of purine nucleotides could lead to a reduced source of energy to the mucosal epithelium and to a reduced capacity for repair of the gut mucosa, which could partly contribute to IBS physiopathology.
WHAT ARE THE CONSEQUENCES IN PRACTICE?
These data suggest a role of the gut microbiota in the physiopathology of IBS with differences between IBS-C and IBS-D. Moreover, these results point towards the potential role of a deficiency in purine nucleotides, in particular via an over-consumption of hypoxanthine by the microbiota and the host cells. These results point the way to treatments stimulating the production of microbial hypoxanthine or inhibiting xanthine oxidase locally in the intestine.
Conclusion
This longitudinal study with integrated multi-omics shows the value of longitudinal studies in humans and highlights functional alterations to the microbiota in IBS which may potentially be implicated in the physiopathology of this disease. The new leads which have been identified may be new therapeutic targets.
Probiotics
Probiotics: essential information for understanding and choosing them correctly.
What exactly are probiotics? They were not “officially” defined until the 21st century. However, consumption of these beneficial microorganisms goes back to time immemorial.
Did you know? Our ancestors took the ancestors of today’s probiotics!1 From Neolithic times, fermentation of certain foods has been seen to hold incredible virtues. Milk, wheat, or vegetables became easier to preserve, tastier, easier to digest… and better for health.1,2
From the beginnings of time, at least 5000 years ago, the Egyptians, Romans and Hindus already appreciated fermented milk,2 and the Turks considered it to be the elixir of life. Is it the secret to their legendary strength? Three centuries before our era, Chinese workmen building the Great Wall of China ate fermented cabbage to keep healthy.2 The famous Hippocrates, “father of medicine” recommended that Olympic athletes eat cheese.1 Even the soldiers of the terrifying Genghis Khan found fermented milk to be a source of vigor that allowed him to win battles!3 But it was not until the beginning of the 20th century, particularly after Louis Pasteur’s work, that we discovered that these virtues were due to beneficial microorganisms.1
What is a probiotic?
Probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”. 4,5
Do you need explanations for this original expert version?
On good form, ready to act: dead microorganisms are not probiotics!
Like bacteria or yeasts.
Just the right amount to act effectively and safely.
In this case, they have a positive effect on the health of the person taking them.6
A probiotic is not…
- … an antibiotic, quite the contrary! The term “probiotic” (for life) was suggested by researchers in the 1960s, as opposed to “antibiotic” (against life).1
- … a microbiota, which describes all the microorganisms present in a given environment - such as the gut. The microbiome, is simply the genome (all the genes) of all these microorganisms!7
- … a prebiotic, consists of special, non-digestible food fibers that specifically nourish the good bacteria in the microbiota and, thereby, are beneficial for health.8 When they are added to probiotics in specific products, they are called symbiotics.9
- … a fermented food, which is a food that has been elaborated with certain living microorganisms and via certain enzymatic transformations—such as yogurt, cheese, fermented cabbage—is not necessarily a probiotic even though it has health benefits.10
- … fecal microbiota transplantation (FMT) is a treatment consisting of treating the microbiota of a sick person by transplanting that of a healthy donor. Currently, FMT is only used in cases of relapsing intestinal infections caused by a bacterium called “Clostridioides difficile”.11
What are probiotic microorganisms?
Commonly called “microbes” or even “germs”, microorganisms are “microscopic” living beings, that means that they cannot be seen by the naked eye.12 They often consist of only one cell!
Microorganisms include bacteria, which are ubiquitous in our environment: they can be found in the earth, water, and even on and in our bodies!12, 13 Microscopic fungi can also be found: yeasts (from Saccharomyces, the baker’s yeast, to Candida, which causes fungal infections) or molds (like Penicillium, which gives Roquefort cheese its “blue” color, but also penicillin, the famous antibiotic).12,14,15,16 Viruses are also microorganisms, but as they cannot survive without infecting a cell, they are still not considered as “living beings”.12,17 There are also amoeba, microalgae, etc.18,19 This little world is actually gigantic: there is already a billion bacteria in a teaspoon of earth! But fear not! Over 99% of them are harmless for humans.12,20
The microorganisms that are most commonly used as probiotics are:
- bacteria from the human microbiota or from fermented dairy products,
(sidenote:
Lactobacilli
Rod-shaped bacteria whose main characteristic is the production of lactic acid, from where they get the name “lactic acid bacteria”.
Lactobacilli are present in the oral, vaginal and gut microbiota of humans, but also in plants and animals. They are found in fermented foods, such as dairy products (e.g. certain cheeses and yoghurts), pickles, sauerkraut, etc.
Lactobacilli are also found in probiotics, with certain species recognized for their beneficial properties.
W. H. Holzapfel et B. J. Wood, The Genera of Lactic Acid Bacteria, 2, Springer-Verlag, 1st ed. 1995 (2012), 411 p. « The genus Lactobacillus par W. P. Hammes, R. F. Vogel
Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004 Jun;70(6):3189-94.
Smith TJ, Rigassio-Radler D, Denmark R, et al. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013 Jun;109(11):1999-2007.
)
(Lactobacillus) and
(sidenote:
Bifidobacterium
A genus of Y-shaped bacteria, most species of which are beneficial to humans. They are found in the gut of humans, and in some yogurts.
They:
- Protect the gut barrier
- Participate in the development of the immune system and help fight inflammation
- Promote digestion and improve symptoms of gastrointestinal disorders Sung V, D'Amico F, Cabana MD, et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics. 2018 Jan;141(1):e20171811. O'Callaghan A, van Sinderen D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front Microbiol. 2016 Jun 15;7:925. Ruiz L, Delgado S, Ruas-Madiedo P, et al. Bifidobacteria and Their Molecular Communication with the Immune System. Front Microbiol. 2017 Dec 4;8:2345. ) (Bifidobacterium); 3,21 - yeasts, such as Saccharomyces boulardii, isolated from the skin of lychees.3,15
All are designated by a specific Latin name
- Their genus comes first, for example Lactobacillus,
- followed by the species within the genus, which gives for example Lactobacillus “acidophilus”,
- and finally, the strain contained within this species, in the form of a series of letters and/or numbers, which like a barcode indicates and precisely identifies the microorganism. The strain differentiates the genetic particulars of each species of microorganism, making each probiotic unique.21
For example, Lactobacillus acidophilus XYZ123
Have you forgotten your Latin?
Think of a fruit salad with plums, cherries, peaches and nectarines. They all come from the same genus of tree, the Prunus. For example, the species Prunus avium gives cherries, while Prunus persica gives peaches - of which there are even more varieties: yellow, white, smooth or fuzzy, round or flat, etc.22
How are probiotics chosen?
Finding the “chosen ones” among the billions of species of microorganisms: that is the hard task that researchers face! Probiotic species often bear the names of their researchers, to commend their efforts: Saccharomyces boulardii was isolated by Henri Boulard23 and Lactobacillus reuterii (which is now called Limosilactobacillus reuteri24) by Gerhard Reuter25.
Regardless of whether they come from milk, fruit, or the human body, potentially beneficial microorganism species are studied from every conceivable angle in order to find the most promising strains.
- The first stage consists in identifying them, following the characteristics of their genome. The microorganisms are classified. They are given a name and a strain number.4,26
- The second stage consists in gradually reducing the potential candidates on the basis of their beneficial properties, such as anti-pathogenic, anti-cholesterol, or bowel-regulating actions.4,26
- The third stage consists in verifying their safety, i.e., whether there are any health risks; researchers therefore check whether they contain antibiotic-resistant genes, toxins, and whether they cause any undesirable effects. They also check whether the candidate probiotics are capable of surviving in certain extreme conditions, such as the gut (temperature, pH, bile acids, etc.).4,26
- The fourth stage is not to be taken light-heartedly. It is when the efficacy of the probiotic is validated in humans, in what are called “clinical trials”, which should follow precise recommendations from the local or national health care authorities. It is at this stage that the tested microorganism can be classified as a probiotic.4,26
- In the last stage, researchers ensure that the probiotic is alive and remains at the effective dose throughout the product’s shelf-life. The probiotics are then stored in an “international microbial strain bank”.4,26
What are probiotics for?
Our bodies contain several microbiota. The most important one is in the gut (that is, the “intestinal flora”), but there is also a microbiota of the skin, the vagina, the mouth, the respiratory tract, etc.28,29 Billions of microorganisms work together within these microbiota.28,30 The majority is harmless or beneficial for health.31 Some are potentially pathogenic; in other words, they could cause diseases, but their development is impeded by “friendly” microorganisms32. For different reasons, such as unhealthy eating, stress, disease, or a course of antibiotics, the equilibrium of the microbiota can be disrupted. This is called “ (sidenote: Dysbiosis Generally defined as an alteration in the composition and function of the microbiota caused by a combination of environmental and individual-specific factors. Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. ) ”.28,30 The composition of the microbiota is changed; it becomes weaker, beneficial microorganisms are less abundant, and pathogens seize the opportunity to colonize the space and multiply…33
Have you heard of "dysbiosis"?
By reinforcing the microbiota, probiotics can help it to maintain or regain its equilibrium, acting on our immune defense system by reducing inflammation, protecting us by attacking (sidenote: Pathogen A pathogen is a microorganism that causes, or may cause, disease. Pirofski LA, Casadevall A. Q and A: What is a pathogen? A question that begs the point. BMC Biol. 2012 Jan 31;10:6. ) or their toxins. They thus prevent or correct the disorders or diseases associated with dysbiosis.34,35,34 Nonetheless, depending on the strain, probiotics have different modes of action, and a specific beneficial effect may not be extrapolated from one strain to another.37
Why take probiotics? What are the benefits? What is in it for me?
Probiotics have several benefits for our health. However, according to the efficacy that each probiotic strain has shown in studies conducted on humans, they have proven their worth in very specific situations.5,38
- Digestive system disorders, such as antibiotic-associated diarrhea,39 C. difficile diarrhea,40gastroenteritis,41 traveler’s diarrhea,42 functional bowel disorders (or “irritable bowel”),43,44 lactose digestion disorders,21 inflammatory bowel diseases (IBDS)45 etc
- Winter respiratory tract infections46
- Skin conditions47
- Urinary tract infections48
- Vaginal infections49
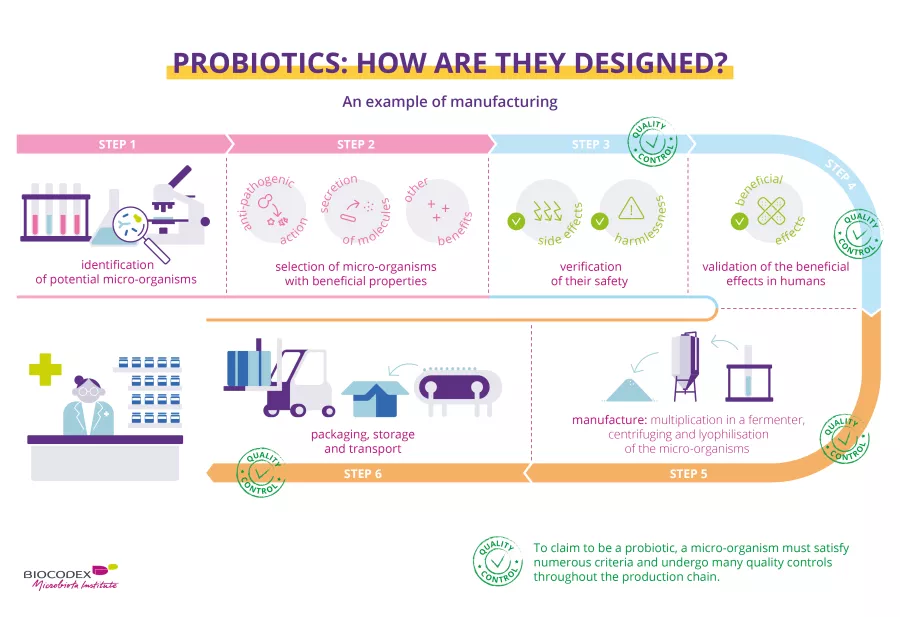
How are probiotics made?
Probiotics are manufactured using a delicate technical process with strict quality controls at every stage of the production line. In fact, they must be produced in a way that guarantees the end product meets all quality and safety standards. The probiotic micro-organisms must stay alive, in a sufficient number and stable until the end of the product’s shelf life, be in the correct dose to produce the stated benefits, and be free from contaminants.5,6,51
This infographic is a simplified and non-exhaustive illustration of the process for selecting and manufacturing probiotics with theoretical examples of quality controls.
Stages of manufacture6,52
This step-by-step process is a simplified and non-exhaustive illustration of the process for manufacturing probiotics.
The chosen micro-organism is cultured with sterilized nutritional substances to encourage it to multiply.
This first culture is transferred into successive (sidenote: Fermenter Device or container in which micro-organisms multiply under controlled culture conditions. Mustafa MG, Khan MGM, Nguyen D, et al. (2018) Omics Technologies and Bio-engineering: Volume 2: Towards Improving Quality of Life, , pp. 233-249. ) (medium-sized, then getting bigger) for industrial-scale production. The growth conditions and absence of (sidenote: Contaminant Unwanted substance, impurity (e.g., pathogenic micro-organisms, residues, etc.) Motarjemi Y, Moy GG, & Todd EC (2014). Encyclopedia of food safety. ) must be closely monitored.
After they have multiplied, the micro-organisms are separated from the culture medium using centrifugation or filtration.
The resulting paste then undergoes fast freezing followed by dehydration to extract any water; this is known as lyophilization, which evaporates at least 96%53 of the remaining moisture. This preserves the micro-organisms whilst ensuring they stay alive.
The “dry cake” produced by the lyophilization process is crushed into a fine powder then mixed (depending on the manufacturer's production methods) with (sidenote: Excipient Substance added to an active ingredient to improve the appearance, taste, or preservation of a medicinal product, or to make it easier to formulate or administer. Cha J, Gilmor T, Lane P, Ranweiler JS. Ch.12 Stability Studies (2011) in Handbook of Modern Pharmaceutical Analysis. Separation Science and Technology, 10 (C), pp. 459-505. ) .
The powder is then packaged in its final form (hard capsules, sachets, ampoules, etc.). The choice and quality of formulation will increase the stability of the product. They are then packaged in their outer container or box.
The packaged probiotics are stored in controlled conditions (e.g., temperature, humidity, etc.).
The batches are then delivered to their point of sale, e.g., a pharmacy.
At each stage of the manufacturing process - even sometimes at several points during a single stage - samples are taken and analyzed to ensure that the product is compliant, in other words to check for optimal quality and purity.6 The micro-organisms must still be alive and safe to consume.6 So that consumers can have complete faith in the quality of their products, some laboratories use not only internal checks but external independent assessors to ensure that the entire manufacturing and quality control process complies with regulations and best practice.27
Choosing the right probiotic among so many is not always easy, as you can see. Not all probiotics are the same. And no probiotic strain or combination of strains will have all of the beneficial effects reported here at the same time.50 Ask your doctor or pharmacist for advice; they will recommend the right products based on your health condition.
International Microbiota Observatory
What does science have in store for us?
There has been a lot of research into human microbiota and probiotics over the past several years. Driven by technological advances in biology, it provides us with new and sometimes surprising scientific insights into the subtle and complex interactions within microbial ecosystems. For precision probiotics, microRNAs, probiotic consortia, postbiotics, etc., research is paving the way for new and promising proposals to meet the current and future health needs of us all.
Science has already revealed some of the secrets behind how probiotics affect our health. We now know that the beneficial effect of a probiotic depends both on the strain and on certain characteristics of the individual consuming it, such as age, diet, state of health, medication and microbiota. 54 As a consequence, this effect can vary between individuals. 55 In recent years, major scientific advances have been made in understanding the interactions between microbiota and the human body. They suggest that modulating these microbial ecosystems could provide new solutions to major nutritional and health issues. 56
So how can we ensure that future probiotics are more effective, more targeted and better adapted to each individual's microbiota? Many teams of scientists have already delved into this vast field of research. The tools they use to gain a better understanding include:
- The omics sciences of today’s biology
(sidenote:
Genomics
Study of all of a person's genes (the genome), including interactions of those genes with each other and with the person's environment.
https://www.genome.gov/about-genomics/fact-sheets/A-Brief-Guide-to-Genomics
)
,
(sidenote:
Transcriptomics
Study of all the RNA molecules within a cell. RNA is copied from pieces of DNA and contains information to make proteins and perform other important functions in the cell. Transcriptomics is used to learn more about how genes are turned on (how they are regulated and expressed) in different types of cells.
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/transcriptomics
https://www.nature.com/subjects/transcriptomics
)
,
(sidenote:
Proteomics
Study of the proteins present in a cell, tissue or organism (proteome), and the structure and function of a protein.
https://academic.oup.com/chromsci/article/55/2/182/2333796
)
,
(sidenote:
Metabolomics
Comprehensive study of all metabolites, i.e., substances produced by biological processes during metabolism, to obtain their specific chemical fingerprint.
Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud. 2015;1(1):a000588.
)
, 56 etc. Together, these disciplines cover all the processes and cellular exchanges involved in the functioning of a body system, such as the human microbiome. In addition,
(sidenote:
Bioinformatics
Computer analysis of biological data
Cunningham M, Azcarate-Peril MA, Barnard A et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021;29(8):667-685
)
enables the enormous volumes of data generated by these new tools to be processed.
- Identifying key microorganisms in microbiota
The scientific knowledge provided by these new methods has enabled researchers to gain a much more detailed understanding of the organization of the microbiota, namely how it works, how the microorganisms interact with each other and with the individual that harbors them, etc. 56 What is the goal? To identify the microorganisms that are key to its balance. In doing this, researchers are able to isolate specific strains and target them for their potential benefits for the microbiota and health. They can also observe the mode of action of these future probiotics within their cells, their interactions with the microbiota and the host's response 54, 57.
- Next generation probiotics
These new scientific and technological approaches have led to the emergence of what is referred to as (sidenote: Next Generation Probiotics (NGP) Live microorganisms identified based on comparative analyses of the microbiota which confer a health benefit to the host, when administered in adequate amounts. Martín R, Langella P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front Microbiol. 2019;10:1047. ) . Among the most recently identified are Roseburia intestinalis, Faecalibacterium prausnitzii, Akkermansia muciniphila. These strains, which come from species that are key for the human microbiota, are thought to act on physiological processes (metabolism, immunity) that are not necessarily affected by conventional probiotics 56 and on certain specific pathological mechanisms involved, for example, in cancer, obesity, diabetes, cardiovascular, inflammatory and auto-immune diseases, pain, etc. 56,58,59,60 They could therefore be classified as (sidenote: Live Biotherapeutic Product (LBP) Biological product containing living microorganisms, such as bacteria, and intended to prevent or treat disorders and diseases (vaccines do not fall into this category). Rouanet A, Bolca S, Bru A, et al. Live Biotherapeutic Products, A Road Map for Safety Assessment. Front Med (Lausanne). 2020;7:237. ) , i.e., medicinal products containing live organisms . Next generation probiotics primarily refer to those strains which do not have the history of use as probiotics. But they may also be previously known probiotics that are genetically modified to produce specific molecules of interest, such as short-chain fatty acids (SCFAs), to increase their survival in the digestive tract and improve their metabolism, immunomodulatory properties, ability to fight certain pathogens, etc. 60
Precision probiotics for personalized medicine
The days of "precision probiotics” are already upon us. And by taking advantage of the variability of the host response, the era of personalized probiotics is dawning! As the concept of personalized medicine is based on factoring in the specific characteristics of each patient in order to overcome the disease, "next generation" probiotics can naturally be integrated into this approach due to their ability to modulate the host microbiota depending on its characteristics. 54 Researchers are now devising ways of analyzing the gut microbiota of anyone who might benefit from a probiotic in order to determine the most suitable one beforehand. The development of miniaturized devices that can be ingested and take a flora sample in order to characterize it is already being considered. Scientists also believe that thanks to the democratization of high-speed sequencing, each of us could soon have access to the genome of our microbiota and thus be able to obtain personalized recommendations for staying in good health through nutrition, probiotics or prebiotics. 54
New public health applications
Probiotics are currently being considered as a response to public health issues, particularly in situations where conventional therapeutic options are lacking. For example, in light of the worrying increase in antibiotic resistance worldwide, probiotics with antimicrobial or immunomodulatory activity are being studied as alternatives to antibiotics. The therapeutic potential of probiotics to treat other major health problems, such as obesity, liver disease, fertility problems, high cholesterol and mood disorders, is also being tested. 56 Lastly, probiotics are thought to help improve cancer treatment as certain strains appear to optimize treatments (and/or reduce their toxicity) for colon, lung and kidney cancers, etc. 60,62
Focus on microRNAs, revealed by transcriptomic analysis
Discovered in the 1990s, microRNAs are of great interest to researchers as these fine regulators of gene expression are involved in many cellular processes. MicroRNA dysfunctions have been linked to a number of diseases, including cancer 63, and they are now being studied as therapeutic targets. 64 They also regulate interactions between the body's cells and the microorganisms in the microbiota.
However, probiotics seem to be able to modify their expression, for example when it is associated with intestinal inflammation. The possibility of using probiotics to act on microRNAs in order to balance the microbiota and treat certain diseases, particularly digestive diseases, looks very promising. 65
Consortia pack an even bigger probiotic punch
Probiotics are also undergoing change as scientists are experimenting with them in groups... and in pieces! The balance of the microbiota depends on the interactions between its microorganisms, and several species may be abnormally underrepresented in the event of dysbiosis. Inspired by the success of (sidenote: Fecal transplantation This therapeutic procedure consists in placing stool from a healthy donor in a patient’s digestive tract in order to restore the balance of their intestinal flora. For the time being, it is only approved for the treatment of recurrent Clostridioides difficile infections. Quigley EMM, Gajula P. Recent advances in modulating the microbiome. F1000Res. 2020;9:F1000 Faculty Rev-46. ) which incorporates an entire bacterial ecosystem, researchers are currently testing consortia of probiotics, i.e., formulations of several strains acting as a 'network' and in synergy 56 to achieve a defined therapeutic effect. 66
Have you ever heard of postbiotics?
Post bio...what? Postbiotics! 67 These are not whole, live microorganisms, as probiotics must be, but microbial fragments, inactivated strains (the cell is dead and no longer multiplying) or bacterial metabolites (proteins, enzymes, etc.) capable of providing benefits to the host. They are beneficial because they are easy to produce and preserve, and they are said to have similar or even superior effects to their probiotic counterparts. 56,68 Further studies are needed to confirm these encouraging initial results.
Bacteriophages: viruses that can kill bacteria....
But that's not all... Research into phage therapy, i.e. viruses that specifically attack certain pathogenic bacteria in the microbiota, is being resumed after decades of dormancy. Recent research suggests that phages could become part of the therapeutic arsenal for fighting multidrug-resistant infections. They could also help to correct the dysbiosis associated with certain diseases or serve as a 'means of transport' for targeted drugs. Further scientific work and clinical trials are once again needed before phage therapy can become one of the new, effective and safe options for tomorrow's medicine. 69
Microbiota's world

Recommended by our community

"That is so, true" - Farida Persaud (From My health, my microbiota)
"Thank you for your important info's!" - Sylvie Lalonde (From My health, my microbiota)
The Biocodex Microbiota Institute is dedicated to education about human Microbiota for General Public and Healthcare Professionals, it doesn't give any medical advice.
We recommend you to consult a healthcare professional to answer your questions and demands
11 Zallot, Camille : Transplantation de microbiote fécal et pathologies digestives, La Lettre de l'Hépato-gastroentérologue, Vol. XXI -n° 1, janvier-février 2018.
13 Site Web Microbiology Society : Bacteria (accédé le 05/06/21).
14 Site Web Microbiology Society : Fungi (accédé le 05/06/21).
17 Site Web Microbiology Society : Viruses (accédé le 05/06/21).
18 Site Web Microbiology Society : Algae (accédé le 05/06/21).
19 Site Web Microbiology Society : Protozoa (accédé le 05/06/21).
20 “Microbiology by numbers.” Nature reviews. Microbiology vol. 9,9 (2011): 628.
22 https://jardinage.lemonde.fr/dossier-212-cerisier-prunus-cerasus.html
28 Site Web Inserm : Microbiote intestinal (flore intestinale) (MAJ 01/02/16, accédé le 06/06/21).
29 Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res. 2012;160(4):258-266.
31 Normal Microbiota and Host Relationships. 3 Jan. 2021
32 McFarland LV. “Normal flora: diversity and functions”. Microb Ecol Health Dis 2000;12:193–207
38 Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67(6):449-458.
BMI-23.38
Celiac disease
Celiac disease is an autoimmune digestive disease caused by a gluten intolerance. Various factors are at work, including an imbalance in the intestinal flora.

Gluten is a substance that is naturally present in wheat, barley, and rye. Unlike a gluten allergy, intolerance appears slowly and can go unnoticed for many years. In Western countries, between 0.7 and 2% of the population is affected.
An often asymptomatic disease
In its classic form, celiac disease starts around the age of six months, after the introduction of the first cereals into the diet. The classic symptoms are chronic diarrhea, lack of appetite, and apathy. However, most often celiac disease is asymptomatic.
A genetic predisposition at fault?
Celiac disease appears in people who have a genetic predisposition to it. Their immune system produces antibodies in the presence of gluten that attack the wall of the intestine. The result is that digestion is altered and nutrients aren’t absorbed as well. Other factors are involved such as the age at which gluten was introduced and repeated intestinal infections. The gastrointestinal microbiota may also play a triggering or aggravating role, according to a hypothesis that is supported by the existence of dysbiosis in these patients. The gastrointestinal flora contains fewer beneficial bacteria and more potentially pathogenic germs in comparison to healthy subjects. A gluten-free diet reduces this imbalance, but cannot fully correct it.
Diagnosis rests on a clinical examination and the presence of indicative signs, along with the search for specific antibodies in the blood and a biopsy (if necessary). Genetic predisposition has been shown through gene study (HLA typing).
Modifying the microbiota as prevention
The only treatment for celiac disease is to remove gluten from the diet. However, another approach – targeting the dysbiosis – has researchers interested. It consists of modifying the gastrointestinal microbiota to prevent the disease from developing, in cases of increased genetic risk, or to improve forms that are severe or even resist the gluten-free diet.
- Maladie cœliaque: de l'enfance à l'âge adulte. Association Française de Formation Médicale Continue en Hépato-Gastro-Entérologie
- Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 2006 ; 101 : 2333-40
- Marasco G, Di Biase AR, Schiumerini R, et al. Gut Microbiota and Celiac Disease. Dig Dis Sci. 2016;61(6):1461-1472.
Cystitis and microbiota
Cystitis is inflammation of the bladder, most often caused by urinary infection. The bacteria Escherichia coli, coming from the intestine, are the primary cause. Women are affected much more than men.

45% Almost 1 in 2 women say they take vaginal douches whereas it's bad for their vaginal microbiota
Cystitis affects 30% of women at least once in their life, with a spike in frequency between the ages of 20 and 30. It is 50 times more common in women than men. Symptoms include a burning sensation when urinating, the pressing need to urinate, and cloudy and unpleasant-smelling urine. It is simple to treat, but reoccurs frequently.
Women the most affected
Certain factors make women more likely to be affected: the shorter length of the urethra, sexual contact, catheters, urinary incontinence, menopause, and pregnancy. Anatomical anomalies and diabetes are also risk factors. Too much or too little intimate hygiene are also implicated in the appearance of cystitis.
An intestinal origin
In 90% of cases, cystitis is caused by Escherichia coli bacteria. These bacteria are naturally present in the intestinal microbiota. They can penetrate into the urethra, travel to the bladder, and multiply there.
Standardized treatment
The normal and most effective treatment for cystitis is antibiotics. However, recurrence is common. Some measures can prevent this (drinking water, hygiene, etc.). Studies have shown the benefit of taking probiotics, such as certain lactobacilli. It should be noted that cranberry may play a role in preventing recurrence of cystitis, but the effect has not yet been clearly demonstrated.
- Cystite-aigue
- Whiteside SA, Razvi H, Dave S, et al. The microbiome of the urinary tract--a role beyond infection. Nat Rev Urol. 2015;12(2):81-90.
- Kölher CD, Dobrindt U. What defines extraintestinal pathogenic Escherichia Coli? Int J Med Microbiol 2011; 301:642-7.
- Beerepoot MA, Geerlings SE, van Haarst EP, et al. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190(6):1981-1989.
- Reid G, Charbonneau D, Erb J, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35(2):131-134.
- Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10(10):CD001321. Published 2012 Oct 17.
Infectious gastroenteritis
Children, teenagers, adults... almost everyone has had gastroenteritis at least once in their life. Although the disease is the world’s second most common cause of death,1 there is no cause for concern, since infectious gastroenteritis can be avoided through hygiene measures and treatment is steadily improving.2 Moreover, restoring the gut microbiota can limit the severity and duration of symptoms.3
The gut microbiota Functional gastrointestinal disorders: a set of diseases defined in correlation with the intestinal microbiota Pr. Olivier Goulet : Gastrointestinal disorders in children, the need to act Travelers’ diarrhea
What is infectious gastroenteritis? What link does it have with the gut microbiota?
A diarrheal disease, gastroenteritis frequently involves severe symptoms which disappear rapidly in most cases.4 Patients display an imbalance of the gut microbiota,5 which may persist after the resolution of the infection, and which has been associated with an increased risk of developing chronic disorders.6
Did you know?
The word “gastroenteritis” comes from the Greek words gastron, meaning “stomach”, and enteron, meaning “small intestine”, in other words, an inflammation of the stomach and small intestine.7
Generally benign in Western countries, infectious gastroenteritis is a leading cause of death in the developing world, particularly among children under 5 years of age.4 It accounts for 10% of all child deaths in developing countries, where it represents a major public health concern.1 The high mortality rates in developing countries8 are often9 linked to the severe dehydration that it causes. The disease is caused by the ingestion of (sidenote: Pathogen A pathogen is a microorganism that causes, or may cause, disease. Pirofski LA, Casadevall A. Q and A: What is a pathogen? A question that begs the point. BMC Biol. 2012 Jan 31;10:6. ) microorganisms, most frequently viruses, in which case it is known as viral gastroenteritis.
Rotavirus is one of the main causes of viral gastroenteritis worldwide and of fatal diarrhea in children in developing countries, despite the availability of a vaccine.10 Other viruses, such as norovirus or adenovirus, may also be to blame.4 Moreover, gastroenteritis can also be caused by bacteria (bacterial gastroenteritis) or parasites, particularly during travel.7 However, the pathogen involved is rarely identified.11
Contamination occurs via contaminated food or water, or directly through physical contact aggravated by poor hygiene.6 Although data on the association between the gut microbiota and gastroenteritis are still very limited, patients display a gut imbalance ( (sidenote: Dysbiosis Generally defined as an alteration in the composition and function of the microbiota caused by a combination of environmental and individual-specific factors. Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. ) ) during infection, regardless of the cause.5,12,13 Studies show that children with severe or complicated viral gastroenteritis, and particularly those infected with rotavirus, display a lower gut microbiota diversity than healthy children.14 Lastly, other studies suggest that changes in the gut microbiota may persist over the long term and could have adverse effects on health, such as contributing to the development of irritable bowel syndrome.15 However, current data on the gut microbiota’s contribution to the development, complications and outcome of the disease require further investigation.
Symptoms at times severe but not long-lasting
In addition to diarrhea (defined as three or more loose or watery stools in one day)6, symptoms may include nausea, vomiting, abdominal cramps, fever or anorexia.16 Symptoms of viral gastroenteritis typically last less than a week and usually improve within 1-3 days.4 Special attention should be paid to children, the elderly and immunocompromised individuals, who are at greater risk of dehydration.4 In some cases, the doctor may order additional tests (stool analysis, blood tests, etc.) to rule out other conditions.4
Antibiotics often ineffective or even counterproductive
Regardless of the cause of infectious gastroenteritis, the treatment is symptomatic.4 For diarrhea, rehydration is the main treatment.6 Antibiotic treatment is not essential and is only justified where a bacterial cause has been established for the diarrhea.11 With viral gastroenteritis, antibiotics may even be counterproductive.11 On the other hand, probiotics, which aim to restore the gut microbiota, may limit the severity and duration of symptoms.7,17
This article is based on scientifically approved sources but is not a substitute for medical advice. If you or your child display symptoms, please consult your family doctor or pediatrician.
BMI 21.19
Obesity
Obesity is a worldwide problem that has tripled in the space of half a century.1 According to the World Health Organization (WHO), 39% of adults aged 18 and over are overweight and 13% are obese.
What if someone were to tell you that tucked away in our intestines, our gut microbiota could affect our appetite, our weight and our capacity for storing fat?2

What is obesity?
Obesity and overweight are most often the results of an imbalance between calories taken in and calories expended by the body – in particular, too much fat or sugar relative to actual energy expenditure.3,4 The resulting excessive increase in body fat can be harmful for health and significantly reduce life expectancy.3 Individuals who are overweight or obese are at greater risk of cardiovascular disease, as well as type 2 diabetes.5,6 Obesity is also linked to a number of cancers (such as liver and uterine cancer).6 Body mass index (BMI) provides an approximate indication of overweight and obesity. WHO considers overweight to equate to a BMI of 25 or more and obesity to a BMI of 30 or above.1
How does the microbiota fit in?
Genetic predisposition, a sedentary lifestyle, sleep deprivation, psychological factors and an unbalanced diet that includes too much fat and sugar are just some of the best known causes of obesity. Sometimes, however, adopting a wholesome lifestyle that combines exercise and a healthy diet is not enough to shed our excess weight.3 So, what's the reason? Recent studies of intestinal flora in obese patients have revealed disruptions in the composition of their gut microbiota, creating an imbalance in the microbial ecosystem defined as (sidenote: Dysbiosis Generally defined as an alteration in the composition and function of the microbiota caused by a combination of environmental and individual-specific factors. Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. ) .7 Did you know? Rodent studies have shown that if the microbiota from an obese patient it transplanted into a mice, it gains weight as well!8 Surprised? These new insights offer hope for the discovery of new treatments. In fact, in obese individuals the microbiota is thought to be generally less abundant and less diverse,9 with fewer “good” bacteria such as Akkermansia muciniphila and bifidobacteria, and more potentially harmful bacteria.8 In such cases, the change in the balance of the microbiota is thought to allow the gut to extract energy from food more efficiently and therefore promotes energy storage in obese or overweight individuals.8
Repercussions beyond the gut
The balance between energy taken in and energy expended lies partly in bidirectional communication between the gut and the brain - what researchers refer to as the gut-brain axis.10 This communication between neurons and bacteria is achieved through “signal” molecules which include “ (sidenote: Short chain fatty acids (SGFA) Short chain fatty acids are a source of energy (fuel) for an individual’s cells. They interact with the immune system and are implicated in communication between the gut and the brain. Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020;11:25. ) ” and “ (sidenote: Neurotransmitters Specific molecules that enable communication between the neurons (the nerve cells in the brain), as well as with the bacteria in the microbiota. They are produced by the individual’s cells and by the bacteria in the microbiota. Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int J Mol Sci. 2019;20(6):1482. ) ”.5,6,11 The gut microbiota uses these messengers to help the brain to regulate energy balance, appetite and the feeling of satiety,6 and also modulates mood and eating behaviour.9,10 It can be seen that gut microbiota and obesity are inextricably linked; when the gut microbiota is impaired, it disrupts the digestion, the body’s defence system and the brain, with which it communicates to manage hunger.9 Like in a closed electrical circuit, the different “devices” are connected to one another and allow information (good or bad) to travel between the gut and the brain.
Can modifying the microbiota help to lose weight?
The solution ought to be straightforward, then: slim down and melt away those extra pounds by looking after your microbiota! The experts will tell you it's not quite that simple, though: it requires an understanding of how diet, probiotics and prebiotics, and faecal microbiota transplant (FMT) influence the ecosystem of our gut microbiota.
- Diet: Diet is of course the number one risk factor for obesity. It is also the main driver of microbiota modulation. Currently, no categorical link has been established between the effect on intestinal flora and the extent of weight loss.12 Conversely, the response to a diet is thought to be the result of the initial composition of our intestinal microbiota.7,11
- Probiotics: Many animal studies show that certain probiotics stand out and are thought to affect metabolic profile, weight gain or satiety in rodents.2,7,13,14 The results in humans are encouraging: while there is not so much data, certain specific probiotics had an impact on weight, BMI, waist measurement, body fat and metabolic profile.2,5,6
- Prebiotics: The results for prebiotics differ in humans, whereas their beneficial effects have been widely demonstrated in the laboratory setting2. Generally speaking, studies show that prebiotics have an effect on satiety,7 but that there is unfortunately no knock-on effect on weight.2
- Faecal microbiota transplant: Other therapies for modulating gut microbiota are being investigated, including faecal microbiota transplant (FMT), a current treatment for recurrent Clostridioides difficile infection. Researchers are evaluating its potential benefit in correcting dysbiosis and in influencing eating behaviour and energy metabolism.7
1 https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
3 Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-298..
12 Seganfredo FB, Blume CA, Moehlecke M, et al. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev. 2017;18(8):832-851.
13 Lucas N, Legrand R, Deroissart C, et al. Hafnia alvei HA4597 Strain Reduces Food Intake and Body Weight Gain and Improves Body Composition, Glucose, and Lipid Metabolism in a Mouse Model of Hyperphagic Obesity. Microorganisms. 2019;8(1):35.
14 Legrand R, Lucas N, Dominique M, et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes (Lond). 2020;44(5):1041-1051.
Stomach Cancer
Colorectal cancer and stomach cancer are two gastrointestinal cancers whose origins are most probably influenced by the intestinal microbiota.
The gut microbiota
Colorectal cancer, the importance of environmental factors
With 694,000 deaths per year around the world, colorectal cancer is the 2nd most deadly cancer. Genetic factors are a significant but minority cause of gastrointestinal cancer, which is most heavily attributed to environmental factors such as sedentary lifestyle, obesity, and, in particular unbalanced diet, which causes intestinal dysbiosis. Furthermore, the hypothesis of an imbalance between harmful and beneficial bacterial species with regard to this cancer is more than likely.
Colorectal cancer doesn’t show any symptoms for a long time, then manifests by persistent or sudden problems with intestinal transit: constipation, diarrhea, the pressing need to go, etc.
Examining the stool for blood and colonoscopy are the two primary methods for detecting colorectal cancer.
Surgery forms the basis of treatment via the removal of part of the colon, sometimes supplemented with chemotherapy or radiation therapy.
Bacteria responsible for 80% of stomach cancers
Although various risk factors have been identified (smoking, diet, family history, genetic predisposition), the primary cause of stomach cancer is Helicobacter pylori a pathogenic bacteria that causes chronic gastritis.
The symptoms are not very specific: stomach pain repeated nausea and vomiting, and a change in general state. Only endoscopy of the stomach and esophagus can confirm the diagnosis.
Surgery is the reference treatment for localized tumors, with partial or total removal of the stomach. For locally advanced forms, physicians also add chemotherapy.
Restoring the microbiota, therapy of the future?
Since the existence of a connection between bacteria and gastrointestinal cancers seems more than likely, manipulating the microbiota with probiotics and prebiotics is being studied as a potential therapeutic treatment.
Type 2 diabetes
Diabetes is a chronic disease related to dysfunction in the production or use of insulin, a hormone that regulates blood sugar. The gastrointestinal microbiota has been identified as one of the environmental factors that may be to blame.

In 2015, 415 million people had diabetes. According to WHO forecasts, it will be 642 million in 2040. Ninety percent of cases are type 2 diabetes.
Insulin resistance, the primary cause of type 2 diabetes
The primary cause of type 2 diabetes is cells in the body losing their sensitivity to insulin. This resistance to insulin makes the hormone ineffective and leads the pancreas to produce more, to the point of exhaustion. As a result, sugar accumulates in the blood, leading to hyperglycemia and serious long-term complications: myocardial infarction, stroke, arteritis in the lower limbs, kidney failure, and blindness.
Involvement of the intestinal microbiota?
Although there is a genetic component to type 2 diabetes, lifestyle is the biggest risk factor for the disease--especially sedentism and a diet including too much fat and sugar. Certain fats and sugars set off an inflammatory response associated with metabolic disorders, which in turn increase the level of inflammation. It starts a vicious cycle, in which the gastrointestinal microbiota contributes because it is unbalanced. Researchers have shown that the gastrointestinal microbiota is disrupted in diabetics, and that this dysbiosis contributes to the disease.
Lifestyle improvements
Above all, diabetes is treated by making lifestyle improvements: weight loss if necessary, regular physical activity, and a balanced diet. It is often necessary to add medication.
The role of certain intestinal bacteria and/or probiotics in diabetes has yet to be confirmed, but their beneficial effects (particularly on appetite and blood sugar) have opened a path for the development of new therapeutic targets for this disease, which affects millions of people.