The vaginal microbiota
The vaginal microbiota: how to take care of it?
Hundreds of bacteria populate the vagina.1 Let’s see how they work and why we should take care of our microbiota!

21% Only 1 in 5 women say they know exactly the meaning of the term “vaginal microbiota”
What is exactly the human vaginal microbiota?
The vaginal microbiota or vaginal flora consists of the hundreds of bacteria and the smaller number of fungi (Candida) that populate the vagina.1
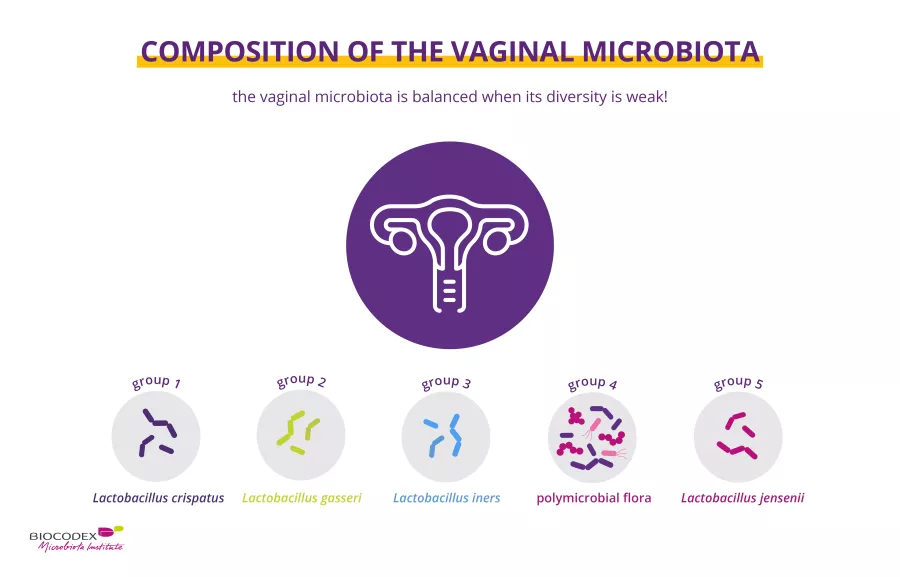
For most women (and in contrast to the gut microbiota) the vaginal microbiota is balanced when its diversity is low (c. 200 bacterial species) and when lactobacilli – rod-shaped bacteria – predominate.1
All women have a vaginal microbiota, but every woman’s vaginal microbiota is different. To date, five main types of vaginal bacterial communities have been described:1,2
-
four are dominated by (sidenote: Lactobacilli Rod-shaped bacteria whose main characteristic is the production of lactic acid, from where they get the name “lactic acid bacteria”. Lactobacilli are present in the oral, vaginal and gut microbiota of humans, but also in plants and animals. They are found in fermented foods, such as dairy products (e.g. certain cheeses and yoghurts), pickles, sauerkraut, etc. Lactobacilli are also found in probiotics, with certain species recognized for their beneficial properties. W. H. Holzapfel et B. J. Wood, The Genera of Lactic Acid Bacteria, 2, Springer-Verlag, 1st ed. 1995 (2012), 411 p. « The genus Lactobacillus par W. P. Hammes, R. F. Vogel Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004 Jun;70(6):3189-94. Smith TJ, Rigassio-Radler D, Denmark R, et al. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013 Jun;109(11):1999-2007. ) (Lactobacillus crispatus, L. gasseri, L. iners, or L. jensenii),
-
one characterized by a low content or absence of lactobacilli
The vaginal microbiota is a dynamic community subject to the influence of factors that include ethnic origin, sex hormones, hormonal contraception, sexual behavior, vaginal douching, diet, smoking habits, social environment (e.g. living space) and genes.1,3
At the same time, the vaginal flora doesn’t live alone. The anus and the entrance to the vagina are located right next to each other and intestinal bacteria from the former can colonize the latter.4 The gut therefore constitutes a natural reservoir of lactobacilli for the vagina, which is important for the balance of the vaginal flora.5,6,7
How does the vaginal microbiota change throughout life?
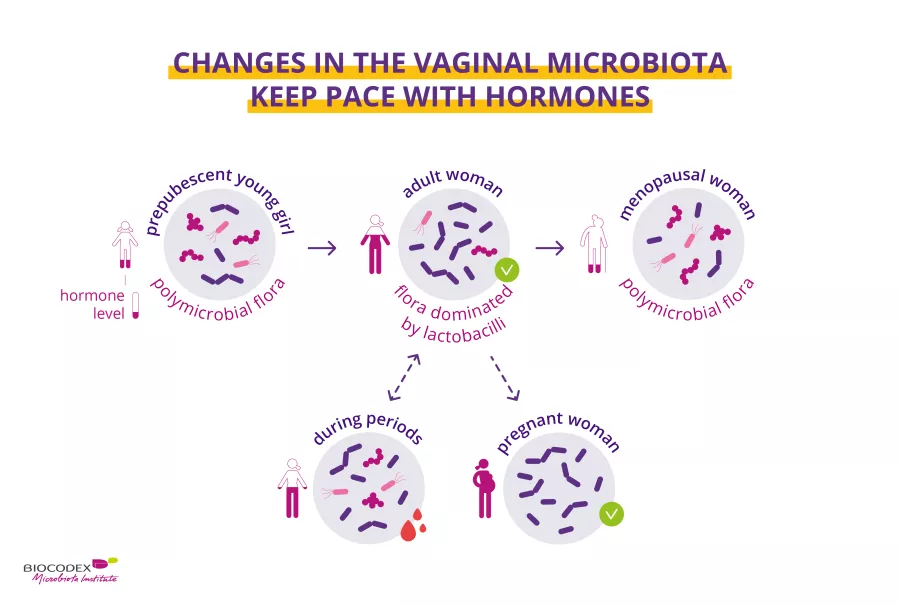
The body evolves throughout life, and so does the vaginal microbiota. The vaginal microbiota’s composition changes greatly from childhood through adulthood to the menopause.1 Hormonal changes alter the rhythm of our lives, and impact the vaginal microbiota as well. For example, menstruation temporarily alters vaginal microbial diversity.8 The microbiota also plays a role in childbirth.1,10 During pregnancy, physiological changes occur to adapt the mother’s body to the fetus and vice versa.9 In pregnant woman, the vaginal microbiota is more stable, less rich and less diverse9, with high levels of estrogen ensuring the outright dominance of lactobacilli.1,8 Lastly, at menopause the vaginal microbiota settles into a new balance.10
Why is the vaginal microbiota a key factor in health?
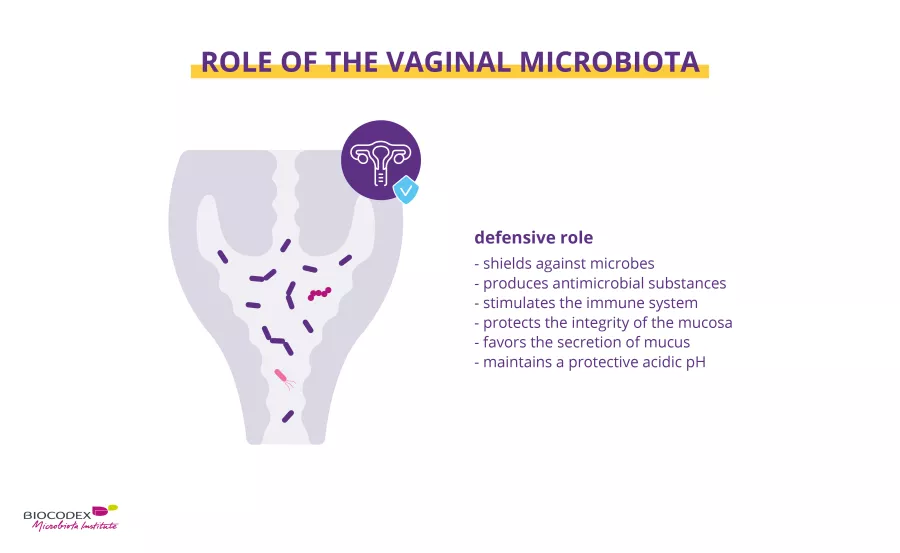
The bacteria in the vaginal microbiota help maintain a healthy vaginal environment.1 Some of these bacteria, particularly lactobacilli, prevent pathogenic (sidenote: Microorganisms Living organisms too small to see with the naked eye. This includes bacteria, viruses, fungi, archaea, protozoa, etc., collectively known as ’microbes’. Source: What is microbiology? Microbiology Society. ) from establishing themselves in the vagina. A number of mechanisms have been proposed:
- by producing lactic acid the microbiota promotes an acidic environment (pH ≤ 4.5) unsuitable for many pathogens11,12
- defensive compounds produced by the microbiota, such as hydrogen peroxide (H2O2) or antibacterial substances (bacteriocins), attack alien bacteria, viruses and fungi11,12
- the microbiota acts as a barrier, making it difficult for pathogens to establish themselves on the vaginal walls. The presence of lactobacilli accelerates the renewal of the epithelium, to which pathogens may try to attach themselves11,12
- the microbiota facilitates the production by the vaginal epithelium of a protective mucus which keeps pathogens at bay11,12
- by stimulating the woman’s immune system, the microbiota improves her ability to fight attacks by pathogens11,12
30% 3 out of 10 women know that the vaginal microbiota is balanced when its bacterial diversity is low.
An unbalanced vaginal microbiota: what are the associated diseases?
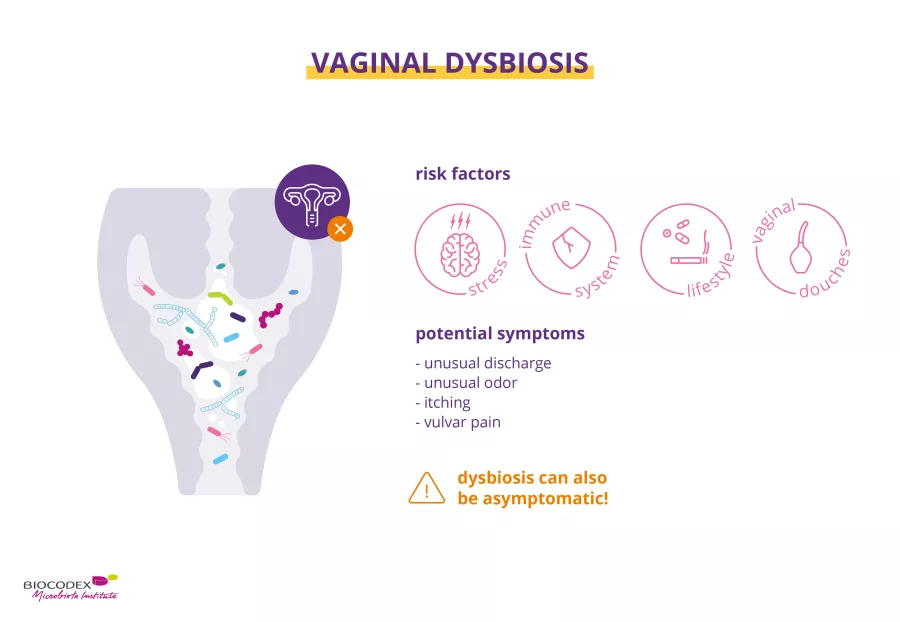
Stress, illness, excessive hygiene (douches), medication (antibiotic therapy, etc.), alcohol, tobacco… all of these factors can impact the composition of the vaginal microbiota.8,13 When the composition of the microbiota is unbalanced, we have what we call a “ (sidenote: Dysbiosis Generally defined as an alteration in the composition and function of the microbiota caused by a combination of environmental and individual-specific factors. Levy M, Kolodziejczyk AA, Thaiss CA, et al. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219-232. ) ”.8,11
A vaginal dysbiosis is when lactobacilli, the key bacteria in the vaginal flora, lose their predominance, paving the way for opportunistic microorganisms to colonize the vagina.8,11 Their presence is often associated with vaginal discharge, itching and burning, or a fishy smell, but can also be asymptomatic.8
A vaginal dysbiosis is associated to:
- a bacterial vaginosis, due to colonization by pathogenic bacteria1
- a candidiasis, due to the proliferation of a fungus8
- reduced fertility11
- a higher risk of premature birth1
Be aware
Bear in mind that women suffering from bacterial vaginosis are more likely to contract sexually transmitted infections (STIs), such as herpes, the papillomavirus, HIV/AIDS, or bacterial infections (gonorrhea, chlamydia, trichomonas).3,14
Knowing what has a direct impact on it, how can we take care of our vaginal microbiota?
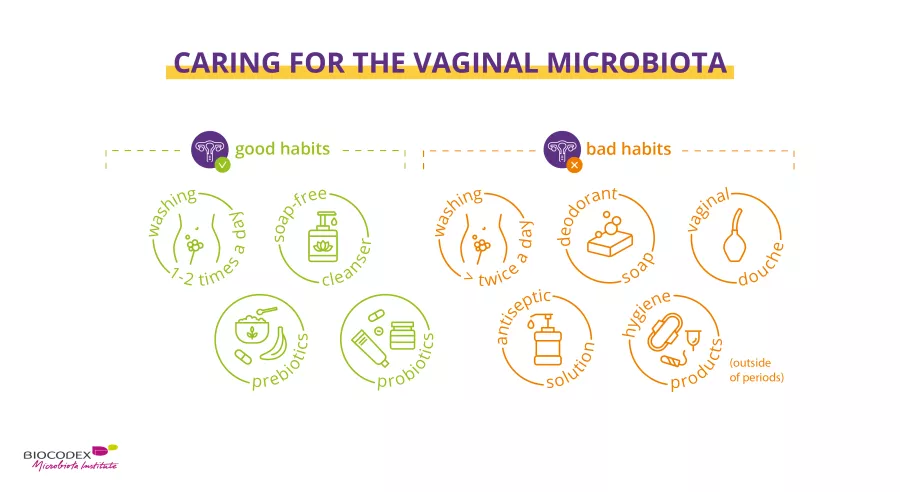
Taking care of the vaginal microbiota is essential. Daily intimate hygiene is crucial to prevent dysbiosis. There’s much false information out there, so it’s important to understand the DO’S and DON’TS.
Daily:
While vaginal douching is not recommended since it alters the vaginal flora, external washing of the vulva with a suitable intimate gel15 helps reduce the unwanted accumulation of vaginal discharge, sweat, urine and fecal contaminants.16
To contribute to the good health of vaginal microbiota:
Hygiene is still necessary15, but it’s not enough. Several options for the vaginal microbiota already exist or are currently being tested:
- Probiotics: Probiotics are live microorganisms that, when administered in appropriate amounts, confer health benefits on the host.17,18 They can reduce or safely correct microbiota imbalances. Probiotics for women administered either via the vagina or orally may help rebalance the vaginal flora, improve symptoms and reduce the risk of recurrence of various vaginal infection13,19,20,21. This is true both for women of childbearing age and postmenopausal women.13,20,21
- Prebiotics: Prebiotics are specific non-digestible dietary fibers that confer a health benefit. They are selectively used by beneficial microorganisms in the host’s microbiota.22,23 When added to probiotics in specific products, they are known as symbiotics.24 Prebiotics for women are thought to promote the growth of lactobacilli and to help restore healthy vaginal acidity.19,25,26
That’s not all!
Recent studies have brought to light other therapeutic options for modifying the vaginal microbiota, such as vaginal microbiota transplantation (VMT). Inspired by fecal microbiota transplants, VMT involves grafting the vaginal microbiota of a healthy woman into women suffering from a vaginal dysbiosis. VMT is a promising option for refractory or recurrent bacterial vaginosis, although to date it has only been tested in a very small number of patients (5 in 2019).27
All the information in this article comes from scientific approved sources. Keep in mind this is not exhaustive. Here are all the studies from which we took all of that information.
International Microbiota Observatory
12 Kovachev S. Defence factors of vaginal lactobacilli. Crit Rev Microbiol. 2018 Feb;44(1):31-39.
15 Bohbot JM, Rica E. Microbiote vaginal, la révolution rose. Editions Marabout. 288 p.