Long considered a source of infection, today microorganisms are often classified as either “good” or “bad”. Is this black or white view appropriate?
Microbes are neither “good” nor “bad”; nor are they our “friends” or “enemies”. We can’t apply this humanized classification to them. Even the most harmless microbe can cause death if the immune system is weakened. However, it is well known that many microorganisms can benefit their host under certain circumstances, whereas others are generally pathogenic.
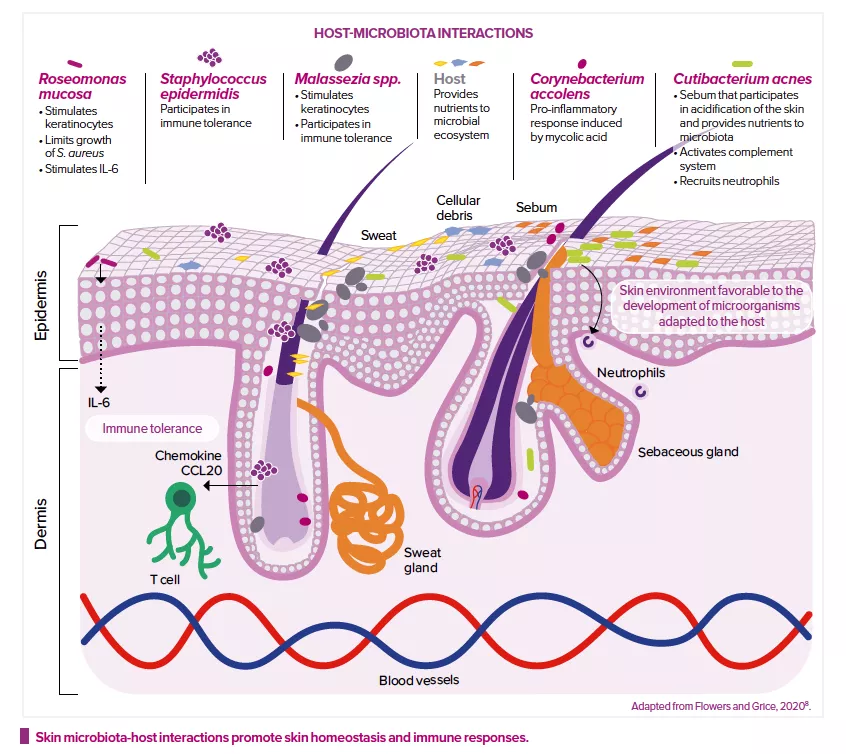
For example, Staphylococci are very abundant on human skin. Staphylococcus aureus has quite a bad reputation: it is often associated with wound infections and several skin disorders, it carries many virulence genes, and its multidrug-resistant form (methicillin-resistant S. aureus, or MRSA) is a major cause for concern in hospital environments. At the same time, numerous recent studies have shown that Staphylococcus epidermidis can stimulate the immune system and the skin’s defenses and even destroy S. aureus biofilms. On the other hand, S. epidermidis is a major cause of implant-related infections and can also become resistant to multiple antibiotics, whereas many people are colonized by S. aureus without experiencing any problems. Therefore, it’s not always a good idea to try to improve skin health by simply lowering the ratio of S. aureus to S. epidermidis on the skin. A good balance between the two should be sought.
Which microorganisms are involved in atopic dermatitis?
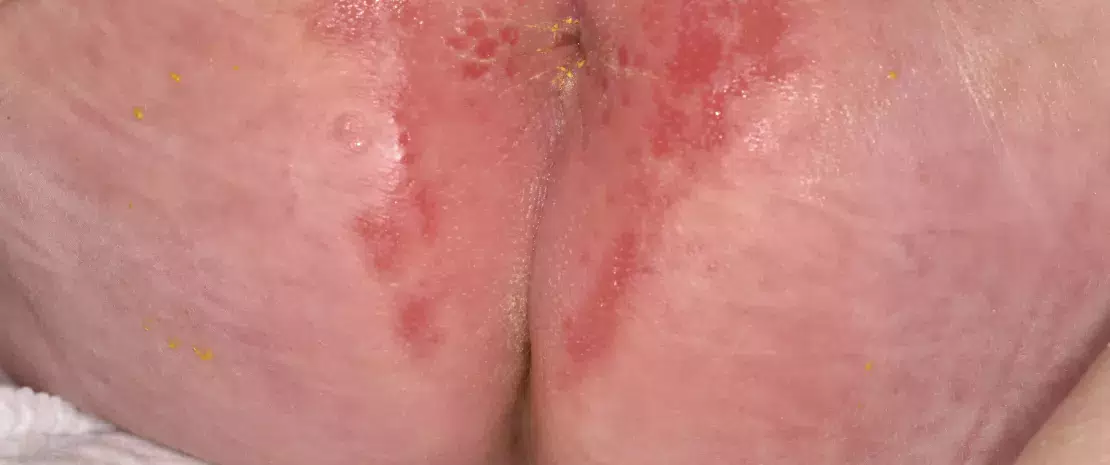
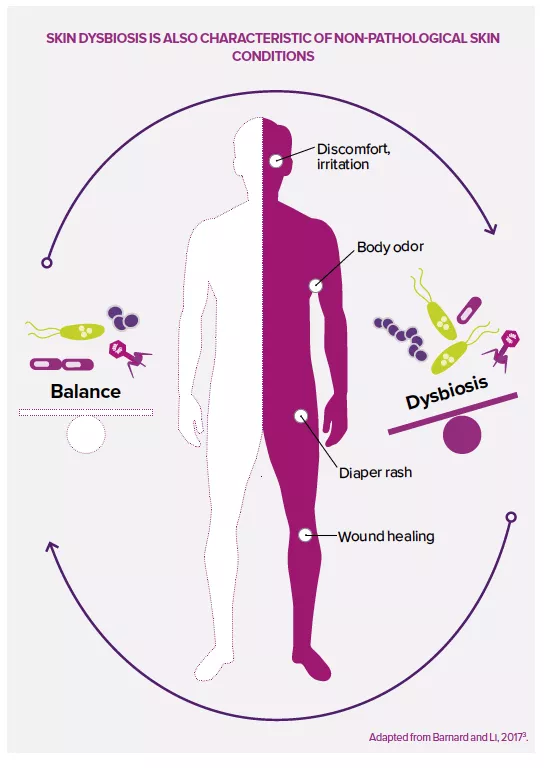
While microorganisms are probably not the main cause of the disease, they make a significant contribution to its pathology. Affected skin areas can be characterized by a microbial dysbiosis: an increased abundance of S. aureus and a reduced presence of typical skin bacteria such as Cutibacterium and Corynebacterium. S. aureus may benefit from a weakening of the skin barrier, possibly the result of altered antimicrobial peptide production in the skin and/or mutations in filaggrin genes1, leading to dryness and cracking of the skin. Inflamed skin is usually treated with antibiotics, which risks causing severe damage to the beneficial part of the skin’s microbiota, as well as antibiotic resistance. Probiotic strategies which aim to increase/restore the abundance of coagulase-negative staphylococci (CoNS) are considered optional and/or complementary.
Can topical and/or oral probiotics prevent or cure skin diseases? What part can they play in therapeutic strategies, now and in the future?
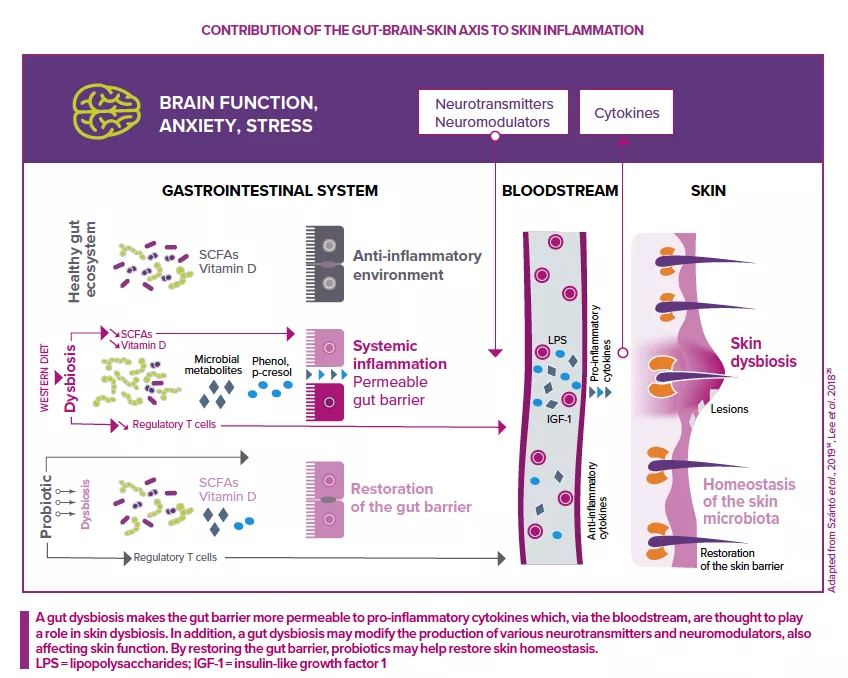
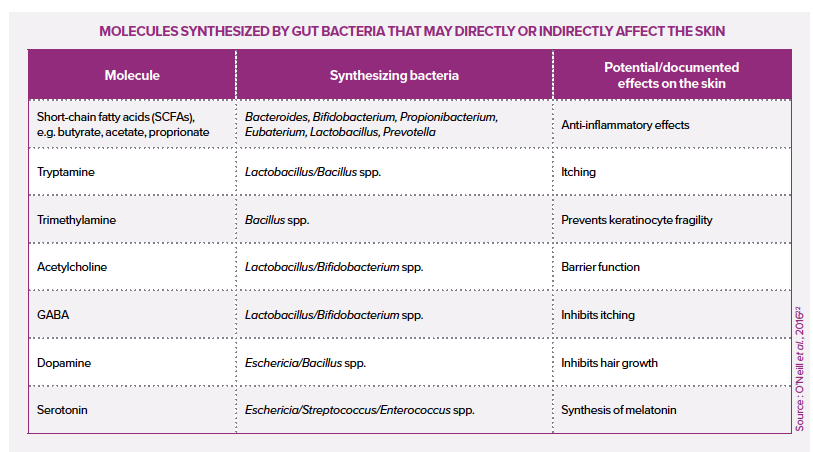
The addition of live microorganisms (probiotics) can certainly benefit the host’s health, for example, by reducing the abundance of pathogens or stimulating the host’s defenses and immune system. Due to the existence of a gut-skin axis, oral probiotics can also have a positive impact on the skin.
However, for most (if not all) major skin diseases,the role of the skin microbiota remains unclear. Although such diseases see marked changes in the structure (community composition) and function (physiological properties) of the skin microbiota, it’s not usually clear whether these changes are the cause or effect of the underlying disease. This is the classic chicken and egg conundrum.
Therefore, in my opinion, it’s a little too early to hope that a simple probiotic cream or capsule can make a significant therapeutic contribution to the prevention or cure of serious skin diseases. Furthermore, research in the gut has shown that, compared to conventional chemical therapies, the effects of probiotics are rather mild and influenced by so many factors that it’s difficult to extrapolate them from highly standardized animal models to humans. Only robust clinical trials could show the effectiveness of probiotics. However, although it’s too early to give a definite opinion for the most serious diseases, to me probiotics seem to be an additional therapeutic option for managing less serious skin disorders and a valuable strategy for improving skincare products. Since it now seems clear that a balanced and diversified microbiota is a characteristic of healthy skin, it makes full sense to preserve and protect such a state, including with probiotic approaches, for example in the case of blemished, sensitive or irritable skin, etc.
Recommended by our community