Gut microbiota #13
By Pr. Markku Voutilainen
Turku University Faculty of Medicine; Turku University Hospital, Department of Gastroenterology, Turku, Finland

Gut microbiota, mediterranean diet and cardiovascular disease
Top contributors of disease burden worldwide, cardiometabolic disease such as cardiovascular disease (CVD) and type 2 diabetes (T2D) have been linked to the individualized nature of the gut microbiome (metabolism and immune interactions). While preclinical studies suggest that gut microbiome and diet engage in a two-way relationship, strong clinical evidence is still lacking especially towards cardiometabolic disease risk. The aim of the study was to examine the interplay of a Mediterranean diet (MedDiet), the gut microbiome and cardiometabolic disease risk in a subpopulation of over 300 men from the long-running Health Professionals Follow-up Study (HPFS). A significant interaction between a healthy dietary pattern and the gut microbiome in relation to cardiometabolic disease risk was identified. This study shows that long-term adherence to a healthy MedDiet was associated with the taxonomic and enzymatic variation of the gut microbiome. Dietary patterns explained 0.7% of the variation which is higher than that caused by antibiotic use. Adherence to MedDiet was associated with the enrichment of microbial degradation of dietary fibers and short chain fatty acid fermentation run by anaerobic fiber metabolizers such as F. prausnitzii and E. rectale. Low adherence to MedDiet with use of red or processed meat was associated with increased microbial synthesis of hepatotoxic secondary bile acids carried mainly by C. aerofaciens. The study underlines the ability of the MedDiet to mitigate cardiometabolic disease risk in the absence of Prevotella copri; while increase in the MedDiet index was associated with decreased myocardial infarct risk in P. copri non-carriers, the P. copri carriers had increased risk. Consequently, individual’s gut microbial profile could be used to tailor dietary interventions to prevent CV disease. For P. copri non-carriers, a MedDiet would be the first-line preventive measure, while P. copri carriers might benefit more from exercise or statins to control CV risk.
Antibiotic prophylaxis and resistance in leukaemia patients
Although antibiotic prophylaxis (AP) can reduce the risk of serious infection in immunocompromised patients the major drawback is antibiotic resistance. Prophylaxis with a broad-spectrum fluoroquinolone may select for antibiotic-resistant microbes and lead to cross-resistance to other antibiotics. In this study, the authors have examined gastrointestinal resistome of children with acute lymphoblastic leukaemia (ALL) to determine the impact of AP on the antibiotic resistance genes (ARGs). Amongst the 49 children with ALL, 31 (63%) received levofloxacin prophylaxis during induction therapy and 18 received no prophylaxis. Trimethoprim-sulfamethoxazole was given for Pneumocystis jirovecii prophylaxis. An increase in the relative abundance of trimethoprim-sulfamethoxazole resistance genes of gut microbiota was detected, which was not modified by levofloxacin prophylaxis. Topoisomerase point mutations of stool bacteria increased during therapy in levofloxacin recipients, but not in the rest of the study population. Levofloxacin target bacterial topoisomerase enzymes that catalyze DNA double-strand break. The increase in the prevalence of fluoroquinolone resistance genes was low and the number of patients with topoisomerase mutations remained small. Although the selective effect of levofloxacin appeared small, an increase in the frequency of fluoroquinolone resistance persisted for at least 2 months after exposure. By contrast, no changes were detected in the aminoglycoside, β-lactam, vancomycin, or multidrug resistance genes after induction therapy suggesting no cross-class resistance to any other antibiotics. In conclusion, fluoroquinolone prophylaxis gives short-term protection against infections but does not increase the risk of cross-resistance to other antibiotics.
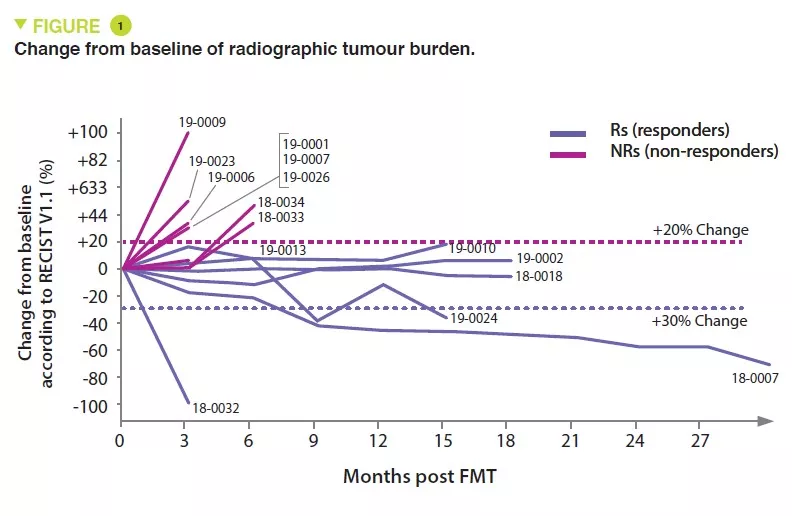
The role of Fecal Microbiota Transplantation (FMT) in melanoma treatment
Immunotherapy to inhibit the programmed cell death-1 (PD-1) checkpoint protein is used for melanoma patients, but only 10-20% obtain complete remission. To increase therapy success, modulation of the gut microbiota has become one of the most promising leads as it shows positive results in preclinical models. However, it has not been investigated in clinical trials. The authors wanted to evaluate immune cell impact of FMT followed by anti-PD-1 immunotherapy in patients with refractory metastatic melanoma. FMT was performed both via colonoscopy and with oral administration of stool capsules followed by reinduction of anti-PD-1 therapy. Stool was obtained from two donors (donor 1 and 2) whose metastatic melanoma had been treated and complete remission reached. No moderate or severe adverse events from FMT were observed. Objective melanoma treatment responses were detected in three patients, they all had received FMT from the same donor (1). One patient achieved complete and two partial remission. After FMT gut microbiota differed from the baseline in all patients and was different depending on the donor (1 or 2). Responders had higher relative abundance of Enterococcaceae, Enterococcus, and Streptocccus australis and lower abundance of Veillonella atypica, but no association between microbial taxa and treatment response was detected. After FMT up-regulation of genes related to the presentation of peptides by antigen-presenting cells (APC) was detected. Responders up-regulated also genes related to APC-activity, innate immunity and interleukin-12. Tumor analysis of all available recipients revealed post-treatment up-regulation of many immune-related gene sets. The study shows that FMT combined with anti-PD-1 therapy is a safe and potentially effective treatment for refractory metastatic melanoma. Modulation of the gut microbiota may overcome resistance to immunotherapy.
Microbiota and breast cancer
In this review, the authors focus on human microbiota throughout life, the links between gut/breast microbiota and breast cancer (BC), and the impact of metabolomics and pharmacomicrobiomics on BC risk and prognosis and treatment choices. While estrogens, high breast density, western- style diet, obesity, alcohol and genetic factors are risk factors for BC, gut microbiota dysbiosis has emerged as a key player in development, treatment, and prognosis of BC through diverse biological processes. β-glucuronidase (BGUS) bacteria modify the enterohepatic circulation of estrogens and may increase the risk of hormone dependent BC. As in the gut, local microbial signatures of breast microbiota in BC patients differ from that of healthy controls. While it is not known whether these findings are a cause or a consequence, a link between breast dysbiosis and BC might exist and is influenced by bacteria and/or their components in local immune microenvironment. (sidenote: Human estrobolome The aggregate of enteric bacterial genes whose products are capable of metabolising oestrogens (Plottel and Blaser, 2011). ) denotes enteric bacterial genes whose products metabolize estrogens. BGUS of intestinal bacteria deconjugate xenobiotics and estrogens leading to reuptake via enterohepatic circulation. Estrogen produced by BGUS may increase the risk of hormone-dependent BC. Other intestinal bacteria metabolize phytoestrogens that may protect against BC. Some gut bacteria produce equol and enterolignans that may diminish hormone-dependent BC risk. Twenty to 30% of western population have microbes (Coriobacteriaceae family) converting isoflavone to equol that has affinity to estrogen receptors, and antiandrogenic and antioxidant activity. Gut free fatty acid receptors are activated by short chain fatty acids and may participate in tumor suppression. Breast and gut microbiota may modulate BC microenvironment: activate aberrant epithelial proliferation, secretion of growth factors, genome mutations, local metabolic microenvironment and angiogenesis. Gut bacteria may inactivate, e.g., doxorubicin and gemcitabine. Gut microbiota has even a double role in radiotherapy efficacy either beneficial and protective or detrimental and resistant. To conclude, in BC patients, microbiota could serve as prognostic and predictive factors of treatment response. In the future, microbiota modulation may improve the outcomes of BC patients.