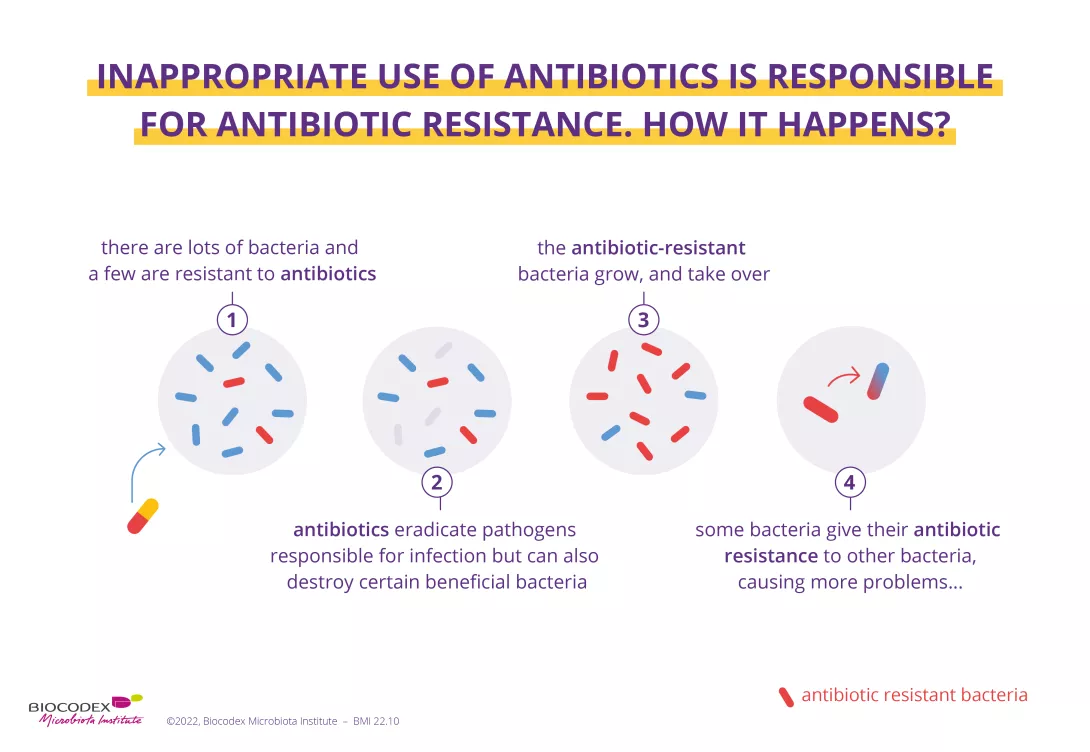
The impact of western diet on the mucus layer
by Dr. Larissa Celiberto

Fiber ingestion helps ensure regular bowel movements. Moreover, since fiber is not digestible by human enzymes, it can also serve as a key nutrient for the gut microbiota as these microbes produce distinct enzymes that are able to ferment and degrade these fibers into important metabolites such as SCFAs.28
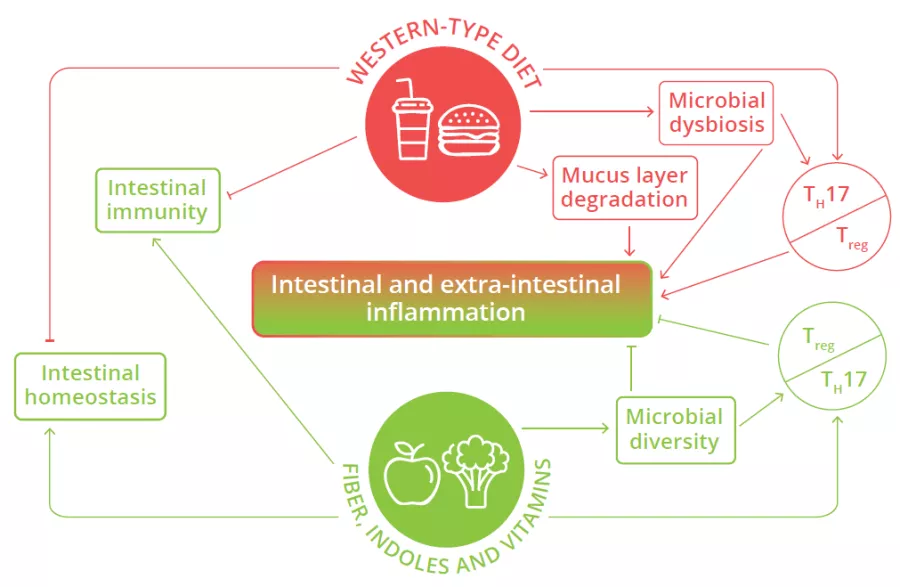
Microbial dysbiosis, mucus layer degradation and alteration of the balance of pro- and anti-inflammatory T cells in the intestine are observed in individuals consuming Western-type diets, leading to intestinal and extra-intestinal inflammation.26
FIGURE 4: Impact of Western diet versus diets rich in fiber and vitamins on local and systemic homeostasis and immunity.
Adapted from Siracusa F et al, 2019.26
The intestinal mucus layer can also serve as an alternative energy source for certain gut microbes (80% of its mass being composed of sugars) when the diet is lacking in fiber.29 This increase in mucus foraging by gut bacteria can prove detrimental as animal studies have shown that mice fed a diet with no fiber are more susceptible to intestinal infections and inflammation. This susceptibility was due to the resident microbiota eroding the mucus layer, such that it could no longer protect the underlying epithelium from invading pathogens.29 Western diets shift the microbiota composition away from fiber degrading bacteria in favor of bacterial species that thrive on mucus (Fig 4).30 Thus, our Western diets may be leading to the loss of protective microbes and the expansion of microbes that weaken key defenses and barriers in the intestine, thereby helping trigger chronic intestinal inflammation.
What is western diet?
Western style diets largely consist of specific dietary fats, sugars and processed foods, environmental pesticides and are lacking in fiber. Consumption of the Western style diet has been linked to obesity as well as inflammatory and metabolic conditions such as type 2 diabetes, insulin resistance and IBD.26 Aside from low quality food with high calories, it is also largely devoid of fiber due its lack of fruits, vegetables, legumes and whole grains, which makes achieving the recommended daily fiber intake of 28–35g27 for adults extremely difficult.