What to take away? Intestinal Immunity
Expert interview: Pr. Brigitte Dréno
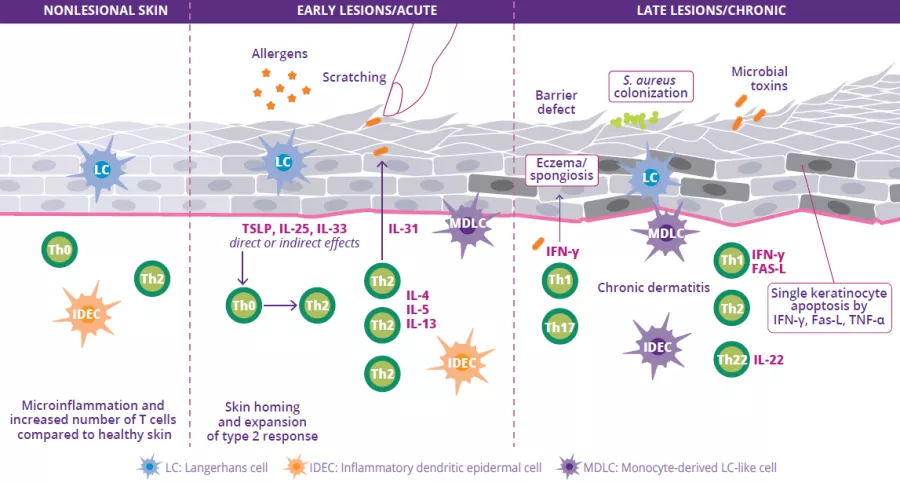
Atopic dermatitis (AD) is a chronic inflammatory skin disease that appears in periodic flareups. Like asthma, hay fever or allergic conjunctivitis, it is classified as an allergic disease. The disease causes very poorly defined oozing red lesions to appear in specific locations on the skin, such as in the folds of the elbow or behind the knees, but at times also on the face or the rest of the body. AD usually appears in early childhood, and may persist into adulthood. The causes are multifactorial and complex and include a genetic predisposition (mutation of the skin protein, filaggrin), an alteration of the skin barrier, a dysbiosis of the skin and gut microbiota, and immune dysregulation.
AD affects 15%-20% of children and 10% of adults in “developed” countries. The number of cases has increased significantly in recent decades due to pollution and contact with allergens.1
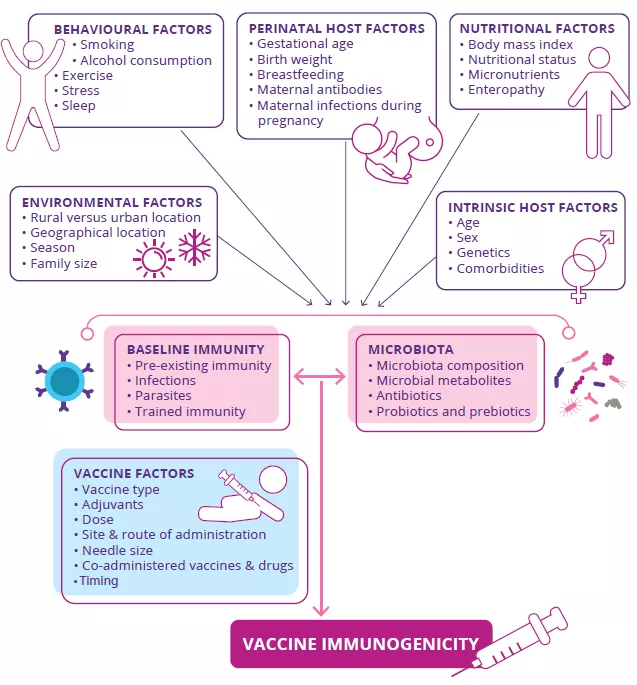
Targeting the gut microbiota to optimize vaccine efficacy?
by Dr Genelle Healey
The hygiene hypothesis and the COVID-19 pandemic
by Dr Genelle Healey
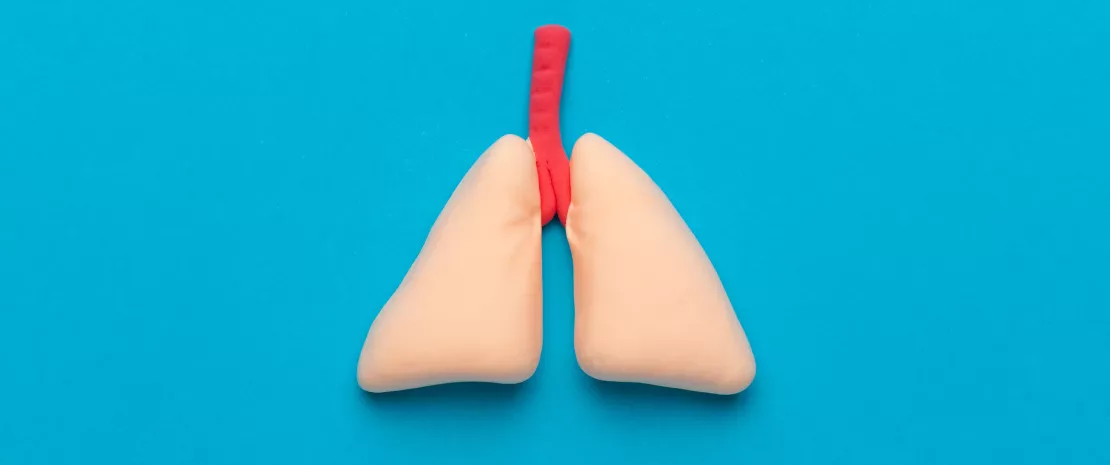
Gut-lung axis in viral respiratory infections
By Dr Genelle Healey
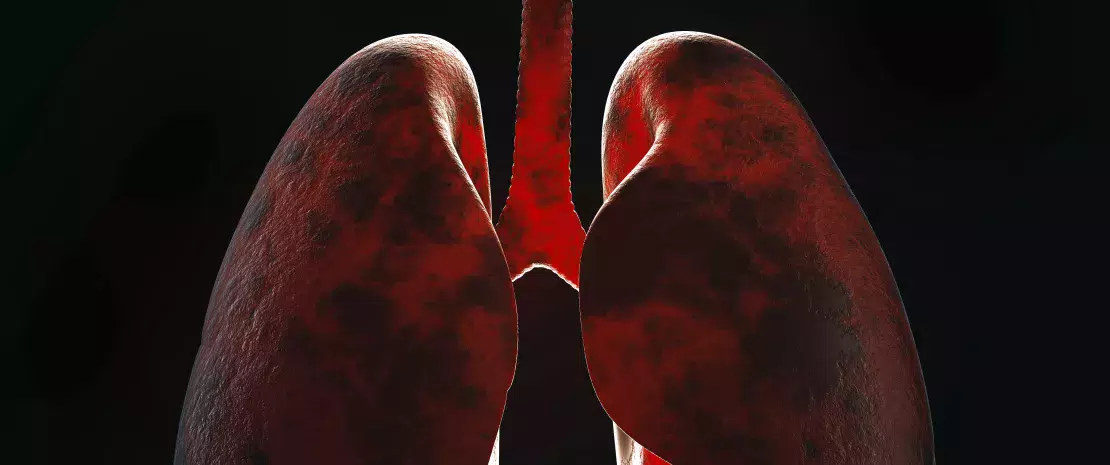
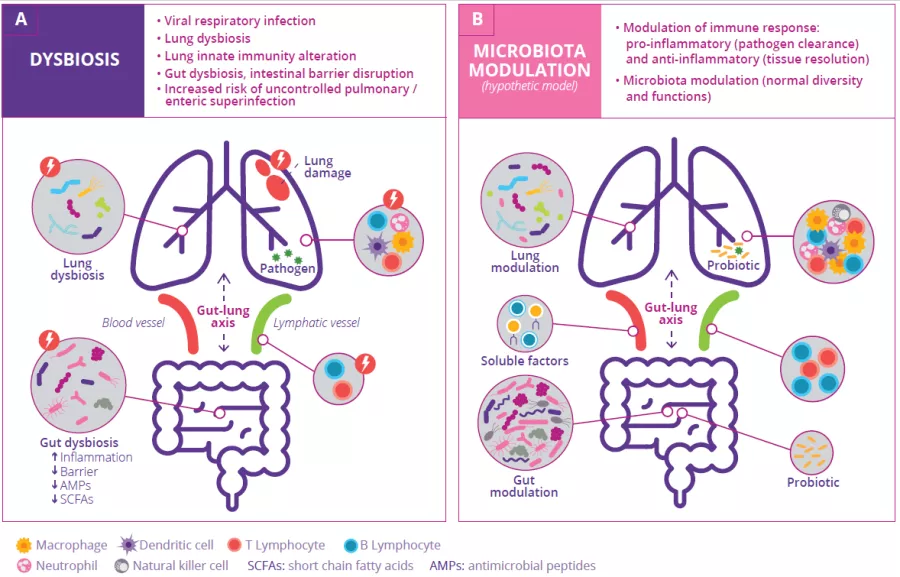
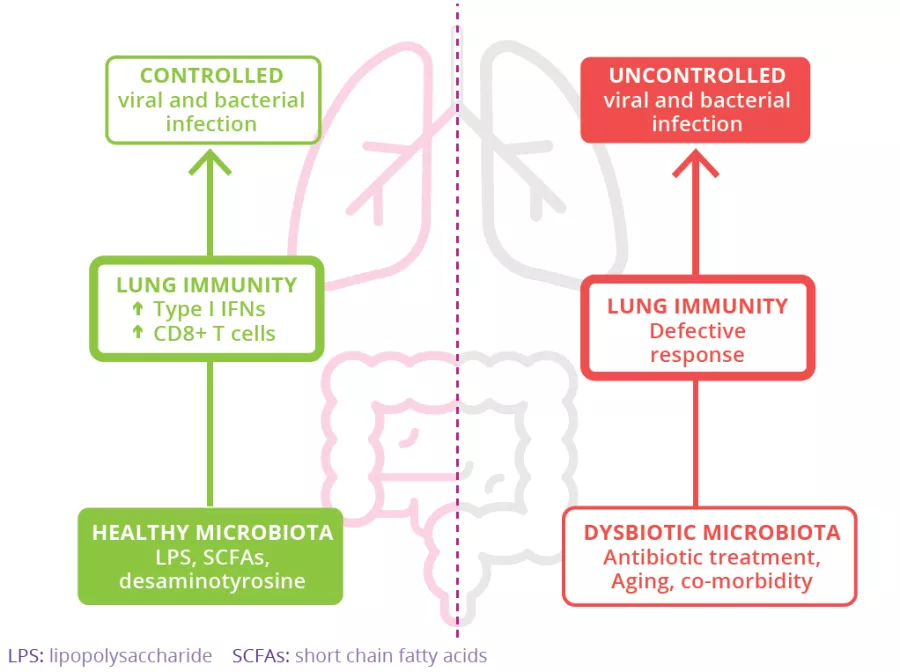
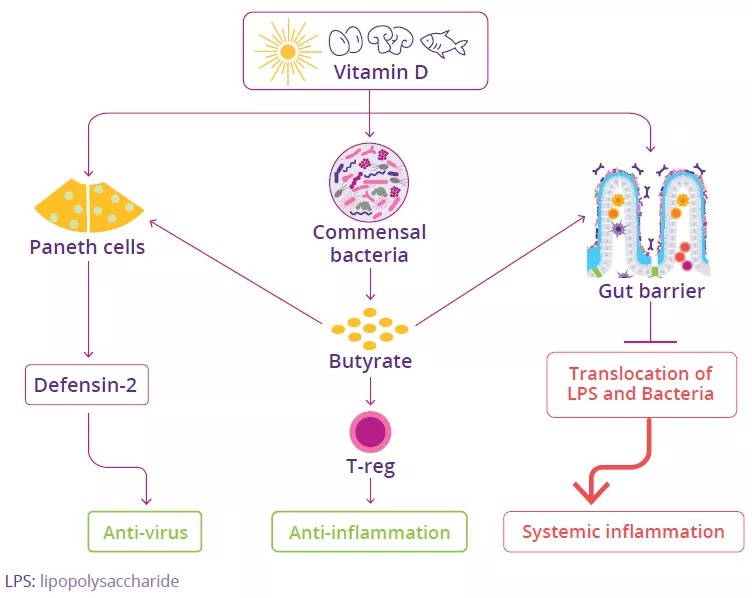
The gut microbiota is involved in the lung’s defense against viral respiratory infections
The microbiota plays a key role in the development, education and function of the immune system, both locally and systemically. While the airway microbiota locally regulates immune function, the gut microbiota can also influence respiratory immunity, via the gut-lung axis.1 Alteration of the lung and gut microbiota has been observed in many respiratory diseases, however whether the dysbiosis at these sites is a cause or a consequence of disease remains to be determined.2 Alteration of gut microbiota composition, through either diet, antibiotic use, aging, or disease, is associated with altered immune responses and homeostasis in the airways,3 highlighting that the gut microbiota can influence disease development throughout the body, including the risk of respiratory infections (Fig 6).4

Dampening gastrointestinal inflammation through nutrition
by Dr Genelle Healey

Irritable bowel syndrome: is fecal microbiota transplantation effective in the long term?
This follow-up study1 confirms that the beneficial effects of fecal microbiota transplantation from a single “superdonor” on symptoms of irritable bowel syndrome and quality of life are maintained one year after treatment.
Antibiotics and neurodevelopmental disorders: does the microbiota play a role?
Antibiotics are often essential to treating certain infections from an early age. But by creating microbiota imbalances, might they influence the development of a child’s nervous system and contribute to disorders such as autism? A recent experimental study1 on mice provides the first – tentative – elements of an answer.
The gut microbiota Autism-spectrum disorders