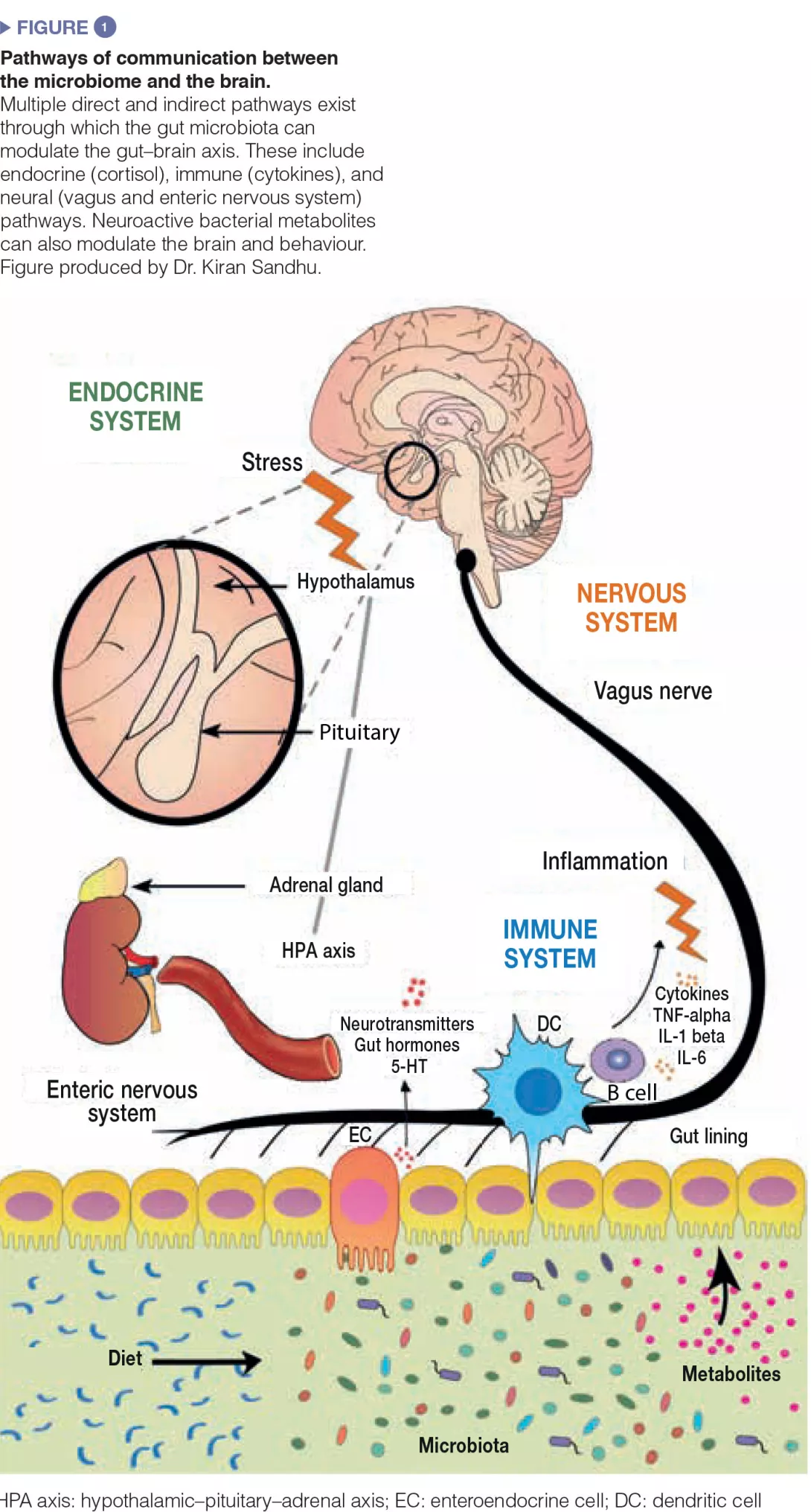
The food additive trehalose increases the virulence of epidemic clostridium difficile
Commented articles - Adults' section
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Comments of the original article by Collins de Collins et al. (Nature 2018)
Clostridium difficile has recently increased to become a dominant nosocomial pathogen in North America and Europe, although little is known about what has driven this emergence. Here the authors show that two epidemic ribotypes (RT027 and RT078) have acquired unique mechanisms to metabolize low concentrations of the disaccharide trehalose. RT027 strains contain a single point mutation in the trehalose repressor* that increases the sensitivity of this ribotype to trehalose by more than 500-fold.
Furthermore, dietary trehalose increases the virulence of a RT027 strain in a mouse model of infection. RT078 strains acquired a cluster of four genes involved in trehalose metabolism, including a PTS permease that is both necessary and sufficient for growth on low concentrations of trehalose. The authors propose that the implementation of trehalose as a food additive into the human diet, shortly before the emergence of these two epidemic lineages, helped select for their emergence and contributed to hypervirulence [1].
What is already known about this topic?
Whole-genome sequencing analysis of C. difficile RT027 strains has shown that two independent lines have emerged in North America between 2000 and 2003 [2]. A comparison with pre-epidemic RT027 strains has shown that the epidemic strains have acquired a mutation in the gyrA gene, that has led to an increased resistance to fluoroquinolone antibiotics. While the development of this resistance has certainly played a role in the spread of RT027 strains, it has also been observed in non-epidemic C. difficile ribotypes and identified in strains from the middle of the 1980s. Thus, other factors have probably contributed to the emergence of epidemic RT027 strains. The prevalence of a second C. difficile ribotype, RT078, has increased tenfold in hospitals and clinics between 1995 and 2007, and has been associated with increased severity [3]. However, the mechanisms involved in the increased virulence remain unknown. Since RT027 and RT078 lines are phylogenetically distant from each other, the changes that have simultaneously led to an increase in prevalence and severity of infection might be due to independent mechanisms.
What are the main results of this study?
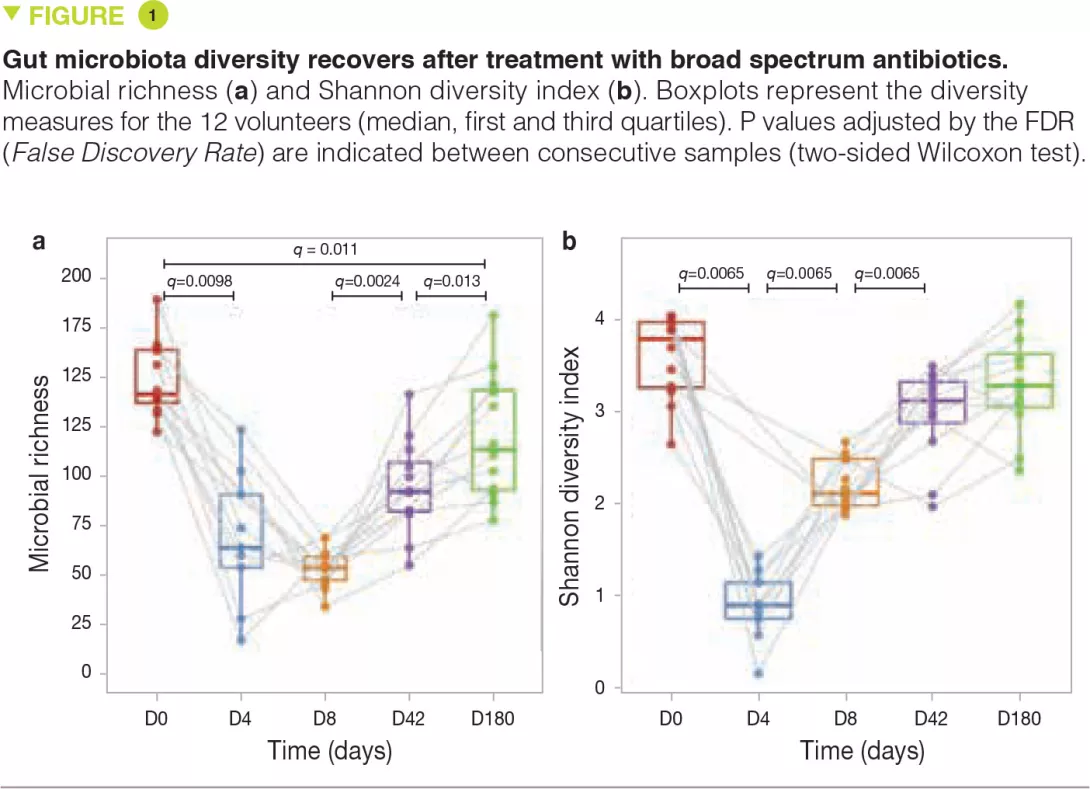
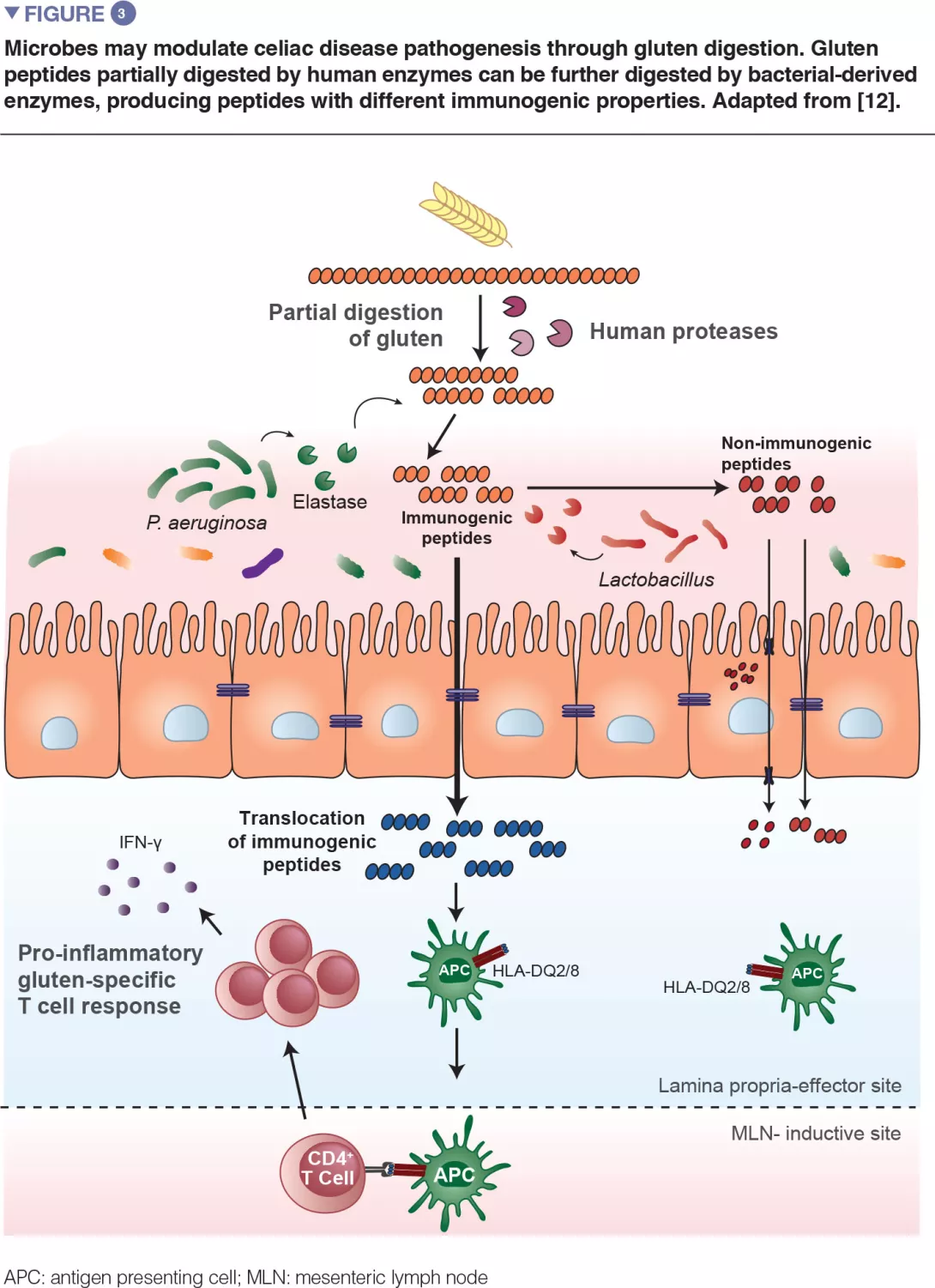
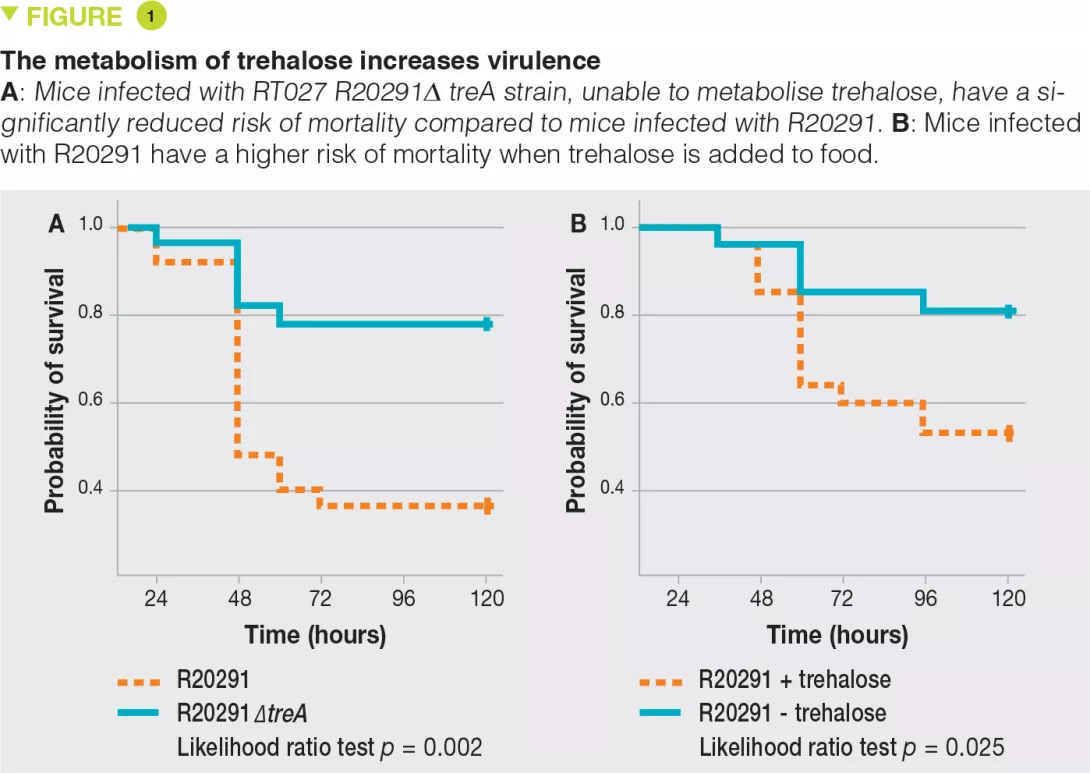
RT027 strains have been shown to have a competitive advantage over other strains both in vitro and in mouse models of C. difficile infection. To investigate the mechanisms involved, the authors examined the use of different carbon sources by the various strains and highlighted an increased capacity of RT027 strains to metabolise the disaccharide, trehalose. By comparing the genomes of many C. difficile strains, the authors identified a putative responsible enzyme, phosphotrehalase enzyme (TreA), which metabolises trehalose-6-phosphate into glucose and glucose-6-phosphate. The authors then observed that this gene was activated in RT027 strains at a concentration of trehalose 500-fold lower than that for the other C. difficile strains. More detailed analyses have identified a polymorphism in the TreA transcriptional repressor (TreR) in all RT027 strains and in other closely related strains responsible for epidemics in Europe and Australia. To determine whether the capacity to metabolise trehalose has an impact on virulence, the authors administered it orally to mice transplanted with human microbiota and infected with either a RT027 strain (R20291) or the same strain deleted for the TreA gene (R20291Δ TreA), and therefore unable to metabolise trehalose. Mortality was much lower with the R20291Δ TreA strain (Figure 1).
Key points
-
C. difficile infection outbreaks with hypervirulent epidemic strains (RT027 and RT078) emerged in the early 2000s.
-
Trehalose is a highly resistant disaccharide that has been used in the food industry since 2000.
-
RT027 and RT078 strains have acquired a competitive advantage that allows them to metabolise trehalose, even at low concentrations, which increases their virulence.
In a second experiment, the authors infected mice transplanted with human microbiota with the RT027 strain (R20291) in the presence or absence of trehalose in the drinking water (given at a dose equivalent to that received during a standard human meal). Mortality was much higher in the presence of trehalose. The two combined experiments confirm the assumption that trehalose in food contributes to the severity of RT027 strains. The genetic analysis of RT078 strains demonstrated an insertion of 4 genes, encoding a second copy of phosphotrehalase (TreA2) and its repressor (TreR2), as well as 2 other related genes. A mutation- and overexpression-based approach confirmed that this insertion was responsible for the capacity of RT078 strains to grow in the presence of trehalose.
What are the practical consequences?
Trehalose is an extremely stable sugar that is resistant to both high temperatures and hydrolysis. Considered ideal for use in the food industry, it has been mainly used since 2000, when a new low-cost production process was discovered [3]. Its use has been authorized in food by the Food and Drug Administration (FDA) in 2000 and by European institutions in 2001. The wide adoption of trehalose coincides with the emergence of infection outbreaks with RT027 and RT078 strains. Overall, the results suggest a causal role for trehalose in food in the emergence of these hypervirulent epidemic strains of C. difficile.
Conclusion
The wide adoption of trehalose in the food industry coincides with the emergence of infection outbreaks with C. difficile RT027 and RT078 strains. These strains have acquired the capacity to metabolise trehalose at low concentrations, conferring them a selective advantage over other strains in an ecosystem in which trehalose has been introduced. This capacity to metabolise trehalose increases their virulence. Overall, the results suggest a causal role for trehalose in food in the emergence of these hypervirulent epidemic strains of C. difficile.