Review of the main contributions related to the intestinal microbiota
Congress review
By Dr Dragos Ciocan
Hepato-gastroenterology and Nutrition, Hôpital Antoine-Béclère, Clamart, France

The Journées francophones d’hépato- gastroentérologie et d’oncologie digestive (French language Hepatogastroenterology and Digestive Oncology Congress) was held in Paris from 21 to 24 March 2019, with an attendance of more than 5,000 French-speaking doctors and researchers. A number of original studies on the intestinal microbiota (IM) were presented.
Faecal transplantation
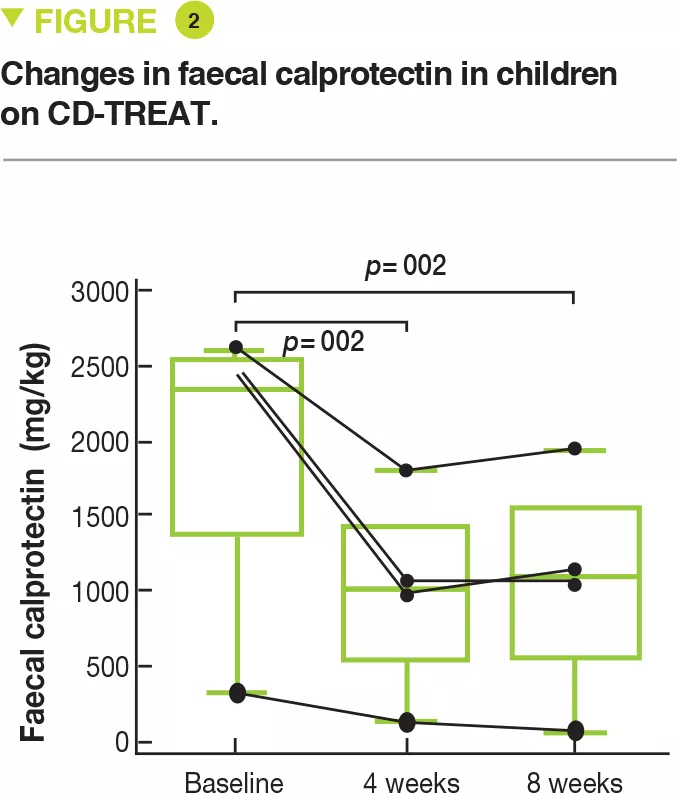
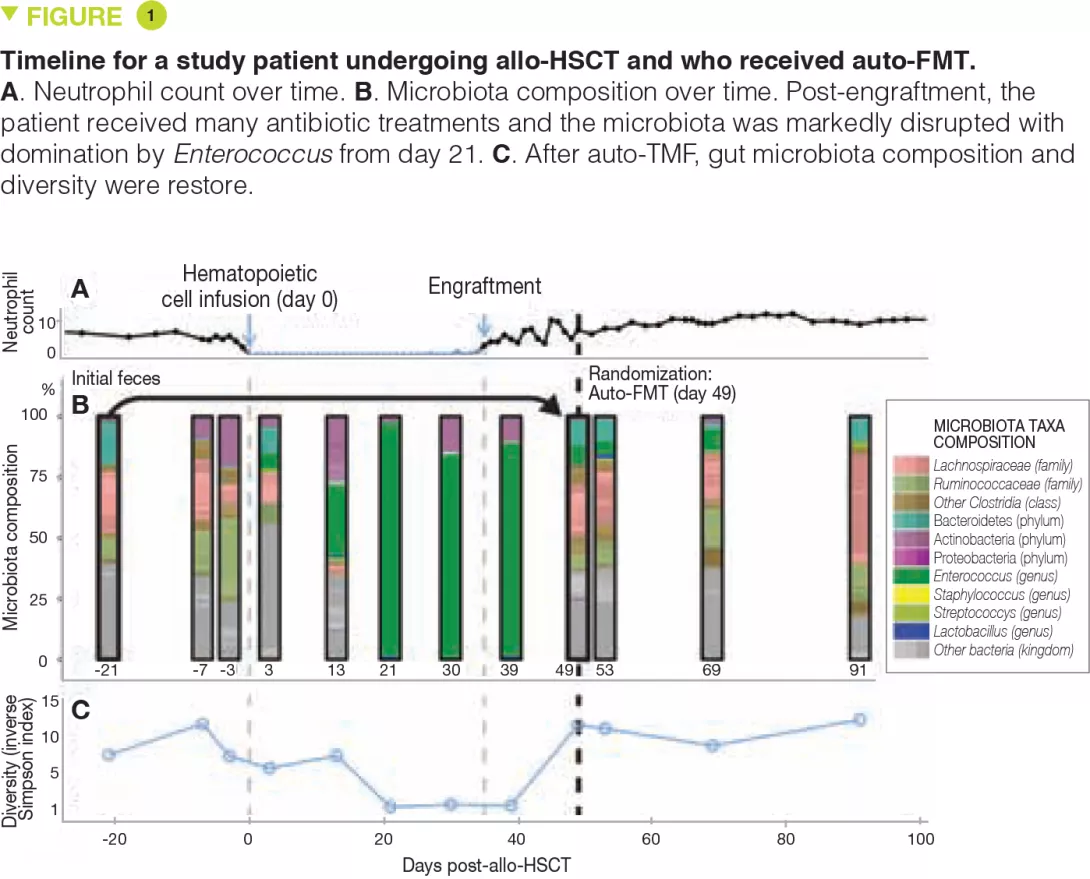
Faecal microbiota transplantation (FMT) is a therapeutic strategy which is used in current clinical practice only for recurrent Clostridium difficile infections [1]. Dr. Eymeric Chartrain presented the experience acquired by the Clermont-Ferrand University Hospital reference centre between 2014 and 2018 on the use of FMT in this indication. FMT was effective in 95% of cases with minor side effects occurring in only 16% of patients. Furthermore, patients reported a significant improvement in quality of life at 6 months post-FMT. The total cost of a FMT intervention is approximately 3,100 euros. Despite this high cost, FMT helps lower health care costs by reducing morbidity and mortality in these patients and is a rational and effective option.
The role of FMT is being studied in many diseases involving the IM, including chronic inflammatory bowel diseases (IBDs). Professor Harry Sokol presented the results of a small, randomized, single blind, placebo-controlled pilot trial in 17 patients evaluating the role of FMT in adults with colonic or ileocolonic Crohn’s disease during a flare-up, who were treated with oral corticosteroids. The primary endpoint – donor IM colonization in the recipient at week 6, defined by recipient IM at week 6 more similar to that of the donor (Sorensen similarity index ≥ 0.6) than to that of the patient pre-FMT – was not reached. Nevertheless, among the secondary endpoints, the FMT group had a reduction in endoscopic disease severity whereas the control group had an increase in inflammation. Colonization by donor IM was associated with sustained remission and patients without donor IM colonization had a recurrence earlier. In addition, the IM composition was predictive of steroid-free clinical remission. Despite the small sample size, this study suggests that FMT could be effective after corticosteroid-induced clinical remission in patients with active Crohn’s disease. Several larger studies including one conducted by Professor Sokol’s group are in progress.
Enterobacteriaceae modulate the effects of fungi in colitis
While the role of the bacterial and fungal IM is known in IBDs, the impact of bacteria- fungal interactions on intestinal inflammation is less clear. Dr. Bruno Sovran presented a study which looked precisely at these interactions in a mouse colitis model. The authors found that administration of a strain of Saccharomyces improved colitis whereas administration of Candida albicans caused it to worsen. However, pre-treatment with colistin, which kills gram-negative bacteria (including proteobacteria) led to a loss of effect of fungi. Administration of colistin-resistant E. coli which restored the enterobacterial population in colistin-treated mice also re-established both the beneficial effects of the strain of Saccharomyces and the deleterious effects of C. albicans on colitis severity. These observations suggest that Enterobacteriaceae are necessary for improved gut colonization by fungi and may explain the effects of some probiotics in colitis.[2]
The gut-brain axis in obesity
It is now well known that the IM plays a role in the pathophysiology of obesity. The IM can also modulate cognitive and psychological functions via the gut-brain axis.[3] Obesity is a risk factor for cognitive impairment, independently of other comorbidities, but the mechanisms are obscure. The MEMOB study, presented by Dr. Sophie Cambos, investigated memory dysfunctions in obese subjects and their correlation with the IM. In this prospective, longitudinal, monocentric study in obese and normal weight subjects, the obese subjects prior to bariatric surgery had memory dysfunctions in comparison with a control population. Analysis of the microbial profile revealed a link between the abundance of Eggerthellales and memory functions: the greater the Eggerthellales abundance, the worse the memory results. These data suggest that obesity – and therefore the associated microbiota alterations – might accelerate cognitive decline via the gut-brain axis.
Microbiota and the liver
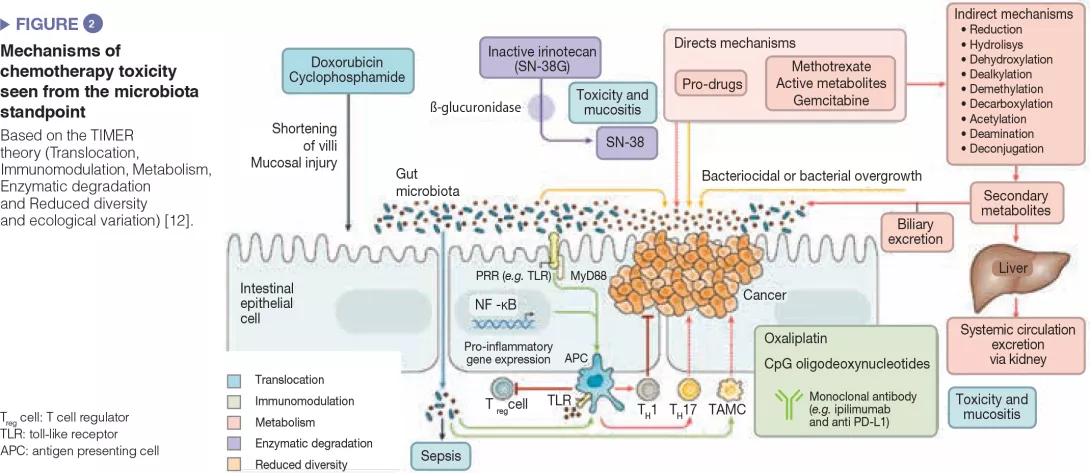
A Biocodex workshop entitled “Microbiota and the liver, from mechanisms to treatment” took place during the meeting. Professor Gabriel Perlemuter reviewed the latest advances concerning the role of the IM in liver diseases. Among the most prominent recent studies, the IM has been found to play a role in susceptibility to developing alcoholic liver disease and non-alcoholic fatty liver disease when using proton pump inhibitors. These drugs promote overgrowth of Enterococcus in the IM, leading to more translocation to liver where it induces liver inflammation.[4] Several pilot studies also investigated the role of FMT in liver diseases (hepatitis B, hepatic encephalopathy and severe corticoresistant acute alcoholic hepatitis) and reported some efficacy in these indications.
Dr. Anne-Marie Cassard discussed the manipulation of the IM in the case of liver disease. She presented data from her group showing that low levels of Bacteroides are associated with the development of alcohol-induced liver injury. Correcting this IM imbalance by administration of pectin, a soluble fibre, prevented and improved the alcoholic-induced liver lesions.[5] However, not all fibres induce the same changes in the IM, even though they have the same beneficial effect on the host.
Conclusion
Furthermore, among the different strategies studied which target the IM and have shown some effectiveness on liver injury (antibiotics, FMT, probiotics, prebiotics), only antibiotics and FMT can induce long-lasting changes in the IM.