Vaginal Microbiota #18
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland


By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland

And what if the harmful effects of Covid-19 in pregnant women required the intervention of the vaginal microbiota? In order to check this hypothesis, researchers conducted a prospective case-control study including 28 non-infected pregnant women and 19 pregnant women suffering from Covid-19. A sample of the vaginal microbiota was obtained with a swab during the active phase of the disease in the month following recovery and evaluated by 16S rRNA gene sequencing. The Covid-19 group displayed significantly greater diversity than the control group. In addition, the Bacteroidetes had gained the upper hand over the Firmicutes, and, at bacterial genus level, the Lactobacillus sp. were significantly less abundant than in the control group. Well, previous studies showed that there was an increased risk of miscarriage or premature birth in pregnant women whose vaginal microbiota were depleted in Lactobacilli. These data corroborate this finding, since 3 women in the Covid-19 group gave birth prematurely (versus 0 in the control group). Despite the small size of the sample, the investigators observed other differences in the composition of the vaginal microbiota in the Covid-19 group. In particular, the women suffering from moderate to severe forms of Covid-19 displayed much higher levels of Ureaplasma spp.: 2.05% vs 0.1% in case of asymptomatic to mild forms. The genus Ureaplasma is involved in different gynecological infections (salpingitis, urethritis, and cervicitis), its over-representation in case of severe Covid-19 also argues in favor of an association of vaginal dysbiosis both with SARS-Cov-2 infection and risks of pregnancy complications. All the more as, in the 3 premature births that occurred in this study, 2 were in the moderate to severe Covid-19 subgroup (n=6). Thus, although this study does not allow the conclusion that a causal relationship exists, these results suggest that Covid-19 may trigger an unfavorable disruption of the vaginal microenvironment in pregnant women. This would be even more pronounced when the infection is severe, and could lead to an increased risk of complications, such as premature birth.
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland

Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder that can be classified to different subtypes: diarrheaor constipation-predominant IBS (IBS-D and IBS-C, respectively), IBS with mixed bowel habits, and unclassified IBS. Many IBS patients benefit from low-fermentable oligo-, di- and monosaccharides as well as polyols (FODMAP) diet. However, only about 60-70% of patients clinically respond to the diet. This study examined the effects of 6-weeks low-FODMAP diet on the gut microbiota in therapy-naive patients with IBS-D. The diet led to an increase in the abundance of Acutalibacter timonensis and Oscillibacter species, as well as a decrease in Bifidobacterium adolescentis, Eubacterium ventriosum, and Clostridium disporicum. Seventy percent of the patients showed improvements in disease manifestations. The authors then studied using ex vivo gut organ cultures how the fecal samples affected gene expression. The post-diet microbiota induced expression of genes implicated in enteric neuronal and muscle functions and suppressed the expression of many genes encoding pro-inflammatory proteins. Gene ontology analysis revealed that post-diet microbiota increased pathways related to extracellular matrix organization, cellular adhesion, and junction assembly. Because many pathways and genes associated with the abundance of B. adolescentis, the authors co-cultured colonic epithelial cells with B. adolescentis and administered mice with the bacterium to find a mechanistic link between the bacterium and gut health. Both in vitro and in vivo, B. adolescentis disrupted epithelial tight junction integrity and gut barrier functions. Ultimately, using in vitro cultures it was found that fructose avoidance under low-FODMAP diet explained the reduced B. adolescentis levels in patients’ postdiet microbiota. The study provides a mechanistic link between diet, microbiome and intestinal functions which will help, in the future, the development of personalized microbiome-based therapies for human diseases.
Little is known about the link between the gut microbiota and resting-state brain activity in patients with chronic insomnia (CI). CI manifests with, for instance, difficulties in initiating or maintaining sleep, obtaining refreshing sleep, and a hyperarousal state. Moreover, CI can impair social, cognitive, and behavioral functioning of the patients. This study investigated associations between the brain functions, gut microbiota composition and neuropsychological performance in patients with CI. The gut microbiota composition strongly associated with neuropsychological performance in CI patients. Specifically, the abundance of Intestinibacter, Lachnospiraceae UCG-003 and Faecalicoccus correlated with the functional connectivity strength (FCS) in the left superior parietal gyrus. This part of the brain is involved in aspects of attention and visuospatial perception, including the representation and manipulation of objects. As expected, the FCS was lower in CI patients than in healthy controls. At the genus level, Alloprevotella, members of Lachnospiraceae family and Faecalicoccus associated with mood and sleep assessment scores. Because Alloprevotella and members of Lachnospiraceae are producers of short chain fatty acids (SCFA), the authors hypothesized that these genera could affect brain functions by modulating SCFA metabolism in CI patients. However, no mechanistic link was established in the study. While the findings of the study were interesting, longitudinal studies are needed to determine whether interventions could affect the gut microbiota of the CI patients and whether the gut microbiota could be targeted, e.g., with probiotic interventions to improve the brain functions in insomnia patients.
Various factors influence infant’s vaccine responses, such as genetics, birth weight, maternal antibodies, and feeding type. Less is known on the role of gut microbiota in immune responses to vaccination though the microbes importantly affect the development of the immune system early in life. This study determined whether the mode of delivery-induced differences in gut microbial colonization patterns in early life are associated with antigen-specific IgG responses to the pneumococcal 10-valent PCV (PCV-10) and the meningococcal MenC conjugate vaccine. Among many variables studied, the mode of delivery and feeding type were the only early life factors significantly associated with IgG responses against one or more serotypes. The diversity of the gut microbiota was not associated with the PSV or MenC IgG responses. The infants, whose gut microbiota was characterized by low abundances of Bifidobacterium and Escherichia coli had the lowest IgG concentrations against both vaccines. Contrarily, anti-MenC IgG concentrations in infants with high abundance of E. coli were ~2-fold higher, which was also associated with vaginal birth. However, at the age of one year, the gut microbiota did not associate with vaccine responses, confirming that early life microbiota is more related to vaccine responses than the microbiota close to the time of vaccination. Regarding the early life gut microbiota, higher abundances of E. coli and Bifidobacterium associated with high anti-pneumococcal responses, while Clostridium, Prevotella and Streptococcus pyogenes associated with low responses. In high anti-MenC responders, higher abundances of many low abundant OTUs belonging to the Lachnospiraceae family were observed. The study proves that understanding the microbial factors driving immune maturation and vaccine immunogenicity is key to improve vaccine performance in children.
By Dr. Nicolas Benech
Gastroenterology and hepatology, Microbiota Study Group, Hospices Civils de Lyon, Lyon, France

The science of the microbiota is a rapidly evolving area and currently encompasses a wide range of scientific and medical expertise, meaning there is now a need to structure and raise awareness of discoveries and bring emerging concepts to the attention of as many people as possible. Gut Microbiota for Health (GMFH) is an off-shoot organisation of the European Society for Neurogastroenterology & Motility (ESNM) whose remit is to promote information and scientific discussion in the area of gut microbiota, especially within the scientific and medical community. Founded in 2012, GMFH organises an annual symposium to gather experts in microbiota science and encourage optimal interactions between both scientists and clinicians. The eleventh edition of Gut Microbiota for Health - World Summit took place in Prague, Czech Republic, on the 11th and 12th of March, and focused on recent developments in innovative treatments targeting the microbiota. A selection of highlights in terms of research and concepts during these two days are presented below.
Gut microbiota research is now developing complex clinical applications such as faecal microbiota transplantation (FMT), next-generation probiotics derived from the human microbiota, medicines developed from microbial products (postbiotics) and also diets based on our current knowledge of host/microbiota interactions. The challenges and issues raised by the arrival in clinical practice of these new forms of medicines today bring up a large number of regulatory, ethical and scientific questions which were developed throughout the congress. At the opening of the symposium, Professor Eugène B. Chang (Chicago, USA) introduced the challenges and new concepts involved in the development of this new type of medicine. Some of these include: the pressing need to establish a specific regulatory framework and design industrial standards capable of underpinning the development of new probiotics; and the need to understand treatments targeting the microbiota in an ecological and dynamic manner, i.e. evolving products that fit into an ecological niche which, in turn, they help to modify.
We have known about the important role played by gut microbiota in modulating anti-tumour immune response for around 10 years; however, the underlying mechanisms are still poorly understood. During the first session of the congress, Dr. Michael Scharl (Zurich, Switzerland) and Professor Harry Sokol (Paris, France) presented their latest findings regarding the identification of microbiological and metabolic candidates for combination therapies with conventional treatments to stimulate anti-tumour immunity. Thus, by studying differences in tumour development in murine models from different animal houses, Dr. Scharl’s team identified four bacterial strains which, when administered alone, reduced tumour development in mice (Eubacterium hallii, Faecalibacterium prausnitzii, Roseburia intestinalis, Anaerostipes caccae) [1].
Interestingly, administration of the supernatant of these strains was sufficient to obtain a stimulatory effect on the anti-tumour immune response.
The metabolism of 3-OH dodecanoic acid has been identified as one of the mechanisms potentially responsible for this effect, paving the way to the development of specific postbiotics.
In line with these findings and the bacterial consortium identified, Professor Sokol presented unpublished work confirming the beneficial impact of Faecalibacterium prausnitzii in the response to immunotherapy.
The re-analysis of metagenomic data from several studies comparing responder and non-responder patients treated with immunotherapy confirmed that the presence of F. prausnitzii was associated with superior tumour response and survival in patients with a dose effect.
In addition to the plenary scientific sessions, several workshops were organised to foster lively exchanges with the experts. Thus, the session on “Engineered microorganisms as therapeutic agents” explored the current advances and perspectives in the development of new genetically engineered microbiological therapeutic agents. During this session, Dr. Nicholas Arpaia (New York, USA) presented an engineered strain of Escherichia coli developed with a lysis cycle coordinated between the different bacteria via a quorum sensing mechanism resulting in the release of a nano-antibody (anti-CD47 antibody fragment) inhibiting an immune tolerance signal in phagocytes [2]. In mice, injection of these bacteria at the tumour graft site resulted in the complete elimination of implanted tumours by the immune system via phagocytosis stimulation but also through adaptive immunity recruitment, thus suggesting the generation of a sustained immune and anti-tumour response. However, the ethical and regulatory framework required to allow the clinical evaluation of this type of treatment has yet to be defined, and this point was specifically discussed during the remainder of the workshop.
Among microbiota-based therapies, FMT is currently the most widely evaluated treatment in clinical practice across many indications. Despite a large number of studies, the factors determining the effectiveness of FMT and its mechanism of action are not yet fully understood. The work presented by Dr. Gianluca Ianiro on the combined analysis of 226 FMTs provided new insights into understanding this therapy by showing that the beneficial effect of FMT was correlated to the engraftment capacity of donor strains in the recipient and that this could be enhanced by the prior administration of antibiotics to open up the intestinal ecological niche, along with the combination of several methods when administering FMT [3].
The beneficial effect of FMT was correlated to the engraftment capacity of donor strains in the recipient.
Several presentations also explored the importance of dietary factors in maintaining the intestinal barrier integrity and its consequences on health. Especially a fibre-rich diet has been shown to prevent the degradatation by Akkermansia muciniphila of the mucus layer that protect the colonic epithelium (presentation of Dr. Mahesh S. Desai, Luxembourg). Conversely, some food additives can boost the penetration of bacteria in the mucous layer in contact with the epithelium and predispose to the development of inflammatory colitis (presentation of Dr. Benoit Chassaing, Paris, France) [4].
The GMFH - World Summit 2023 provided an opportunity to put the major advances in recent years into the development of therapies based on microbiota science into perspective, providing solid guiding principles that are still being confirmed and further refined.
This better understanding of the mechanisms underpinning the efficacy of microbiota- targeted therapies and the complexity of their use in clinical practices illustrates the need for clinical experts able to develop and use microbiota-based applications in the routine care. Dr. Ianiro, a world expert in FMT suggested that such qualifications should be grouped together under the concept of «microbiome clinician ».
At this 11th congress, the GMFH symposiums, by creating a rich and accessible space for exchange between clinicians and researchers, contribute to the emergence of this type of expertise.
1. Montalban-Arques A, Katkeviciute E, Busenhart P, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 2021; 29: 1573-88.e7.
2. Chowdhury S, Castro S, Coker C, et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med 2019; 25: 1057-63.
3. Ianiro G, Punčochář M, Karcher N, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nature Medicine 2022; 28: 1913-23.
4. Chassaing B, Compher C, Bonhomme B, et al. Randomized controlled-feeding study of dietary emulsifier carboxymethylcellulose reveals detrimental impacts on the gut microbiota and metabolome. Gastroenterology 2022; 162: 743-56.
COMMENTED ARTICLE - Children’s section
By Pr.Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France

Rural environments and microbiota are linked to a reduction in the prevalence of allergies. However, the mechanism underlying this reduction is unclear. The authors assessed gut bacterial and fungal composition in urban and rural children in Southern China (EuroPrevall-INCO cohort). The bacterial and fungal composition of airborne dusts from homes in the city and countryside (including mattress dust) as well as dust from henhouses (rural environment) were analysed by 16S rRNA sequencing. Mice were repeatedly exposed to intranasal dust extracts and evaluated for their effects on ovalbumin (OVA)-induced allergic airway inflammation. It was found that children in rural areas had fewer allergies and unique gut microbiota with fewer Bacteroides and more Prevotella. Dusts from rural environments contained a higher level of endotoxins and diversity of bacteria and fungi, whereas indoor urban dusts were enriched with Aspergillus and contained a higher number of potentially pathogenic bacteria. Intranasal administration of rural dusts before OVA sensitisation reduced respiratory eosinophils and blood IgE level in mice and also led to a recovery of gut bacterial diversity and Ruminiclostridium in the mouse model. Faecal microbiota transplant restored the protective effect by reducing OVA-induced lung eosinophils in recipient mice. These results support a cause-effect relationship between exposure to dust microbiota and allergy susceptibility in children and mice. Specifically, rural environmental exposure modulated the gut microbiota, which was essential in reducing allergy in children.
The prevalence of allergic diseases has increased dramatically. It has been shown that children living in the countryside are less prone to asthma than those living in cities. Indoors, dust is the main source of bacteria and fungi. Its composition reflects the outdoor environment and is influenced by external activities (e.g., agricultural), building materials and animals.
The establishment of gut microbiota during the first 1,000 days of life shapes the subsequent development of allergic diseases. Some well-known factors influence the composition of the gut microbiota of infants including antibiotics, delivery method and diet. Any resulting dysbiosis is thought to increase the subsequent likelihood of developing allergic diseases. In contrast, breastfeeding and vaginal delivery protect against the subsequent development of allergic diseases. The intestinal microbiota of these types of infants is characterised by a predominance of bifidobacteria, especially Bifidobacterium breve. Decreased contact with nature was seen to favour intestinal dysbiosis with a dysregulation of Th1/Th2 immune balance in favour of Th2, which is the adaptive immune response involved in allergic diseases (Cukrowska Nutrients).
The mechanisms by which gut dysbiosis in early life influences the development of allergies and asthma are little understood. Farm dust and bacterial lipopolysaccharide are known to induce endotoxin tolerance, thus reducing allergic asthma.
The authors compared urban and rural environmental exposures in China in a human and mouse study. The EuroPrevall-INCO human cohort included 5,139 urban and 5,542 rural school-age children. The prevalence of food allergies and especially asthma, rhinitis, and eczema were higher in urban children (p < 0.001).
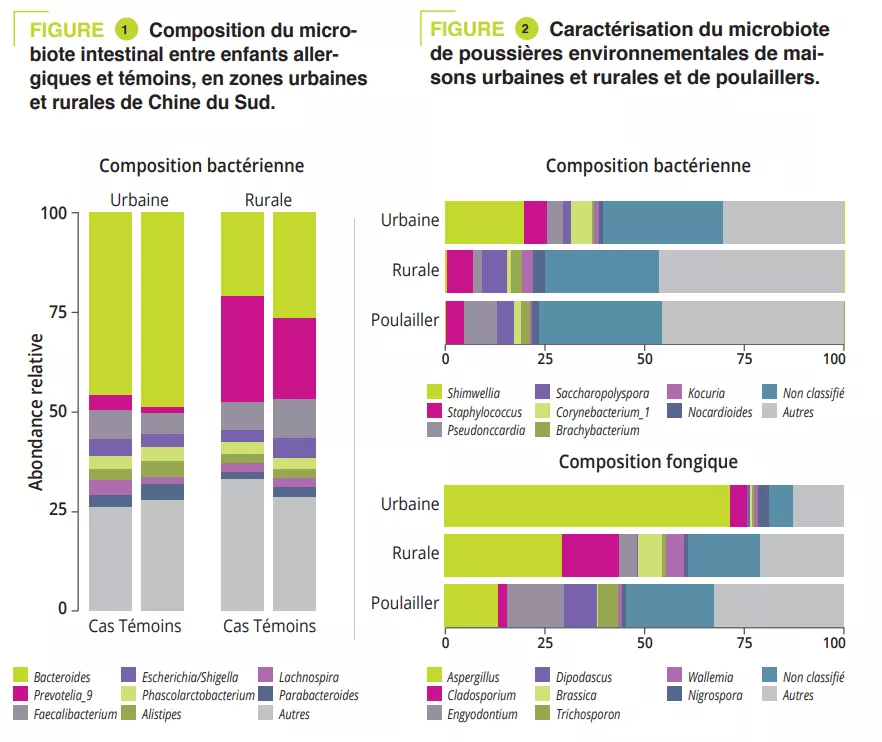
A case-control study included 225 children: 151 urban and 74 rural children. The gut microbiota of all children was analysed via 16S rRNA sequencing and metabolic pathways were assessed via shotgun sequencing. Clinical data and allergen sensitizations were collected. The Prevotella-to-Bacteroides ratio was significantly higher in rural children (p < 0.001). This difference was due to Prevotella_9 accounting for 25% of amplified variants in rural children and <5% in urban children (figure 1). However, no significant difference was observed in gut microbiota composition between cases and controls in both urban and rural participants. The analysis of metabolic pathways identified 14 different pathways between urban/rural participants and nine between controls/ cases. Among these, the L-lactate producing pathway was strongly associated with allergy and pathways involved in sugar degradation and lipopolysaccharide synthesis were abundant in the microbiota of control children.
To mimic the microbial exposure of children in urban and rural environments, mattress dusts were collected from ten urban and ten rural families and dusts from five henhouses from rural families (on the assumption that these may contribute to the microbial environment in rural families). Enterobacteriaceae and Rhizobiaceae were predominant only in urban house dusts (figure 2). A significantly higher a diversity and endotoxin content was observed in rural house dusts compared to urban house dusts and henhouse dusts. Finally, urban house dusts had a significantly higher proportion of potentially pathogenic bacteria. In addition, Aspergillaceae dominated in urban house dusts, whereas Trichocomaceae (genus Penicillium) was more abundant in rural house dusts (hence the higher diversity) and henhouse dusts (figure 2).
To test the impact that exposure to environmental dusts may have on allergic disease by altering the gut microbiota, the researchers exposed mice to dust by intranasal exposure (OVA-induced allergic model). Prior exposure to house dust in rural areas attenuated allergic inflammation (eosinophilic infiltration of airways, in bronchoalveolar lavage (BAL), increased sIgE). Mice exposed to rural dusts showed the lowest increase in the proportion of potentially pathogenic bacteria. The abundance in the gut of Bacteroidales increased and while Clostroidales (including species belonging to both Lachnospiraceae and Ruminococcaceae families) decreased in control mice exposed to PBS as well as those exposed to urban dusts. Finally, the relative abundance in the gut microbiota of Bacteroides and Ruminiclostridium was correlated to eosinophils in BAL (r = 0.59 and p = 0.001 and r = – 0.45 and p = 0.05 respectively).
Early modulation of the gut microbiota, targeting the beneficial effect of rural house dusts could prevent the development of allergic diseases.
This study reported differences in the composition of dust microbiota between urban and rural areas in China. These modulate differently the gut microbiota and its immune response in allergic diseases.
COMMENTED ARTICLE - ADULTS’ SECTION
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France
Fungal microorganisms (mycobiota) comprise a small but immunoreactive component of the human microbiome, yet little is known about their role in human cancers. Pan-cancer analysis of multiple body sites revealed tumour-associated mycobiomes at up to 1 fungal cell per 104 tumour cells. In lung cancer, Blastomyces was associated with tumour tissues. In stomach cancers, high rates of Candida were linked to the expression of proinflammatory immune pathways, while in colon cancers Candida was predictive of metastatic disease and attenuated cellular adhesions. Across multiple GI sites, several Candida species were enriched in tumour samples and tumour-associated Candida DNA was predictive of decreased survival. The presence of Candida in human GI tumours was confirmed by external ITS sequencing of tumour samples and by culture-dependent analysis in an independent cohort. These data implicate the mycobiota in the pathogenesis of GI cancers and suggest that tumour-associated fungal DNA may serve as diagnostic or prognostic biomarkers.
Cancer is one of the leading causes of death worldwide. The tumorigenesis, progression and treatment-response of cancer are influenced by various interactions between the immune system of the host and bacteria in the microbiota. However, the role of fungi (mycobiota) in these processes remains largely unexplored. Fungi and bacteria co-colonise the gastrointestinal tract, skin epithelium, airways and reproductive organs of mammals, forming a complex ecosystem of microbe-microbe and host-microbe interactions with significant implications for human health. While fungal infections account for over 1.5 million deaths worldwide each year, they only account for 0.1% of microbial DNA in the gut, suggesting a disproportionate influence of species from this kingdom on the overall gut microbiome and host immunity. Whether referring to viruses, bacteria or fungi, an ever growing body of scientific evidence suggests a link between the human microbiome and cancer and its outcomes. Several cases showing the association between bacterial species and cancer development/progression have been observed in recent years. Helicobacter pylori is responsible for approximately 75% of the risk attributable to gastric cancer, while genotoxic Escherichia coli, Bacteroides fragilis, Streptococcus bovis/gallolyticus and Fusobacterium nucleatum have been implicated in colorectal carcinogenesis [2]. The common feature of these bacteria is their ability to trigger chronic inflammation, a feature considered to contribute to their tumorigenic capacity. Recent reports have also identified intracellular bacteria in many types of tumour [3].
The mycobiome plays a key role in the activation of innate immunity in the gut. Mycotoxins and bioactive amines have been associated with carcinogenesis. Recent experimental studies support fungal involvement in cancer in some contexts [4]. Sequencing data from tumour banks have revealed the presence of microbial sequences, although the fungal component has not yet been explored.
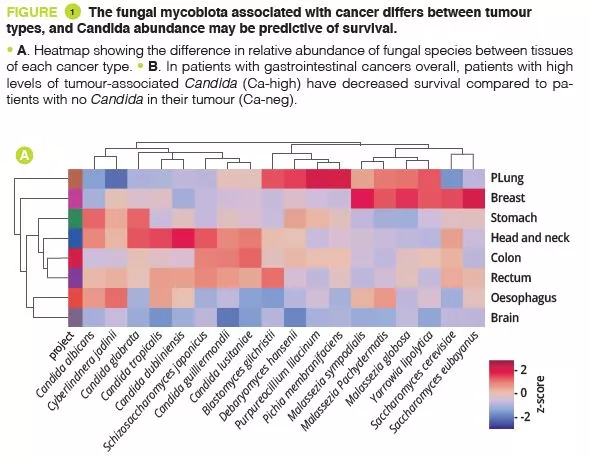
By analysing several types of cancer using “The Cancer Genome Atlas” (TCGA), the authors extracted tumour-associated mycobiome profiles with a species-level resolution. After eliminating contamination and false-positive signals, the authors reported that fungal compositions varied according to the type of cancer, and that some fungi were tumour-type specific, in both gastrointestinal and non-gastrointestinal locations (figure 1A). Overall, up to one fungal cell per 104 human tumour cells were observed, a rate consistent with the finding that fungi make up 0.1-1% of the microbiome, while bacteria are estimated to make up less than 1% of tumour cells [2, 3]. Abundant numbers of several species of Candida, Saccharomyces cerevisiae and Cyberlindnera jadinii have been found in gastrointestinal tumours, while Blastomyces and Malassezia species are abundant in lung and breast tumours, respectively. The authors then showed that several Candida species are alive and transcriptionally active in the tumour. Finally, the abundance of some fungi within the tumour could predict host tumour gene expression, disease status and survival (figure 1B), although these findings still need to be confirmed. Overall, these results suggest an involvement of fungi, especially Candida, in the pathogenesis of gastrointestinal cancers but also highlight their potential as a therapeutic target and prognostic tool.
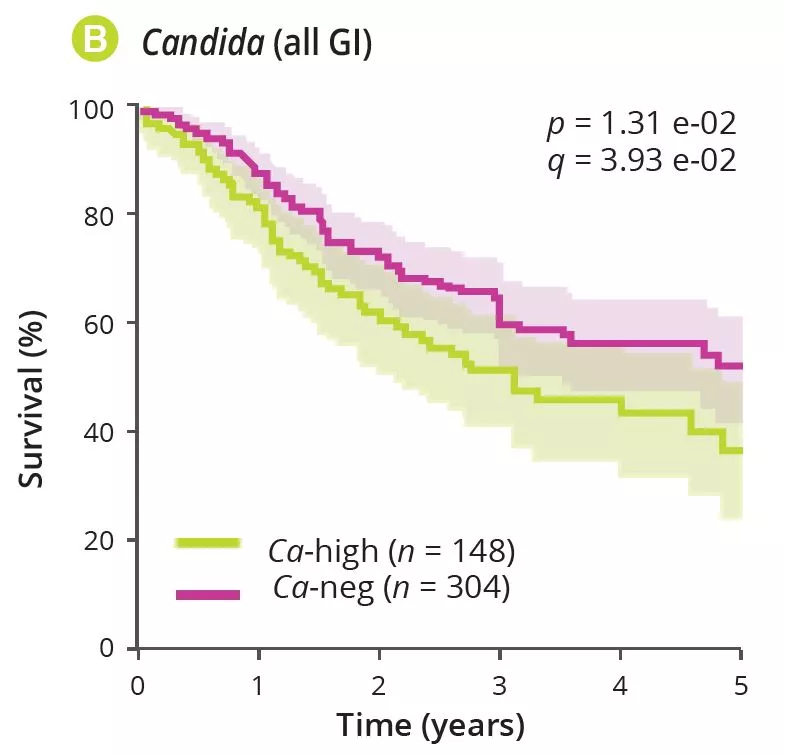
Alongside bacteria, this study reported the presence of fungi in many gastrointestinal and non-gastrointestinal tumours, with some degree of specificity across tumour types and a potential for predicting severity. These results suggest that fungi play a role in the cancer process and its severity. They could also pave the way for the development of new biomarkers or new cancer treatments targeting the fungal component.
An analysis of multiple gastrointestinal and non-gastrointestinal tumours identified tumour-associated fungi, especially Candida enrichment in gastrointestinal cancers. Fungi may also play a role in carcinogenesis. Tumourassociated fungal DNA could serve as a prognostic marker in this context and fungi could represent a new therapeutic target in cancer.

"Interesting developments. Thank you for caring about humanity." -sturehp (From Biocodex Microbiota Institute on X)
"Tumour-associated fungal DNA may serve as diagnostic or prognostic biomarkers. Very interesting. I am curious to see what comes out of this." -Just me. (From Biocodex Microbiota Institute on X)
1. Dohlman AB, Klug J, Mesko M, et al. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022 ; 185 : 3807-22.e12.
2. Sepich-Poore GD, Zitvogel L, Straussman R, et al. The microbiome and human cancer. Science 2021 ; 371 : eabc4552.
3. Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020 ; 368 : 973-80.
4. Alam A, Levanduski E, Denz P, et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 2022 ; 40 : 153-67.e11.
By Dr. Jay Patel
Usher Institute of Population Health Sciences and Informatics, University of Edinburgh, United Kingdom
Although the co-evolutionary role of the human microbiome as a determinant of human health is increasingly recognised in modern medicine, the oral microbiome remains a largely siloed factor contributing to general health and well-being. In health, the oral microbiome maintains a careful symbiotic equilibrium with the host, with harmful bacteria at clinically inconsequential levels. However, external environmental pressures readily turn the oral microbiome dysbiotic, where an improper proportional and diversity of microbes colonise the mouth. These environmental pressures are often highly modifiable risk factors. An expanding body of evidence suggests that the relevance of this disturbance is not merely confined to local disease activity but has a disseminated risk profile for other major chronic diseases of the body, with a high global burden of disease including diabetes, atherosclerotic cardiovascular disease, and rheumatoid arthritis.
In health, the oral microbiome represents a carefully balanced, diverse community protecting the mouth from disease. Modern lifestyle choices can readily upset this balance, rendering the community less protective and increasingly harmful.
The morphology, warmth and moisture of the mouth affords the oral microbiota a highly diverse habitat for colonisation and growth. From birth, children acquire a simple oral microbiome, and with age, the eruption of teeth, and the additive role of external factors, this community becomes increasingly complex. Both host-derived and microbially-derived factors maintain the homeostatic equilibrium of the oral microbiome required for health.
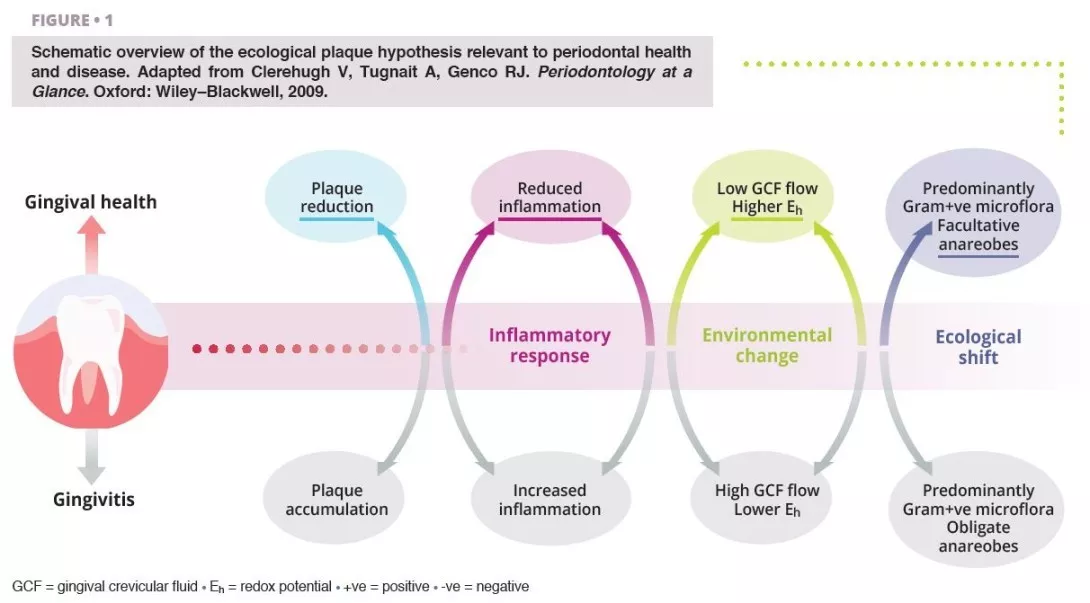
Poor oral hygiene can be a profound ecological pressure that steers complex microbial communities in the mouth into dysbiosis [1].
Ecological shifts in a dysbiotic ecosystem favour the colonisation and proliferation of pathogenic oral bacteria (figure 1). When these species increase in quantity, the risk of oral disease significantly increases. Periodontal disease is a chronic, non-resolving, inflammatory process leading to tissue breakdown of the tooth-supporting apparatus, and can lead to tooth loss, if untreated. Routine activities including chewing, flossing, and toothbrushing can induce bacteraemia, which facilitate haematogenous dissemination of oral bacteria and inflammatory mediators, inducing systemic inflammation in some patients [2]. Patients with periodontal disease––the sixth most prevalent condition affecting humankind globally [3]––show micro-ulcerated sulcular epithelia and damaged periodontal tissues, and thus seem more susceptible to bacteraemia. Therefore, the inflammatory state from periodontal disease metastasises to other sites of the body, which can occur at clinically-relevant levels. Good oral hygiene is therefore essential for controlling the total bacterial load in the mouth, maintaining or re-establishing the oral symbiotic equilibrium, and preventing the dissemination of oral bacteria to other sites in the body.
The characteristics of the oral microbiome are not only confined to oral pathological changes but can influence systemic health, and in cases, this influence is measurable in both positive and negative directions.
The strongest evidence supporting a bidirectional role between oral and systemic health exists for the dose-dependent relationship between the severity of periodontitis and complications arising from diabetes.
Type II diabetes is a metabolic disorder characterised by an insufficiency of insulin production and the subsequent inability for the body to metabolise glucose, leading to elevated levels of blood glucose (chronic hyperglycaemia). Severe periodontitis strongly influences glycated haemoglobin (HbA1c) and fasting blood glucose levels in people with and without diabetes [4]. Periodontitis is thus recognised as the sixth major complication of diabetes, as the risk for periodontitis is raised by 2-3 times for people with the condition [5].
19–33% Compared to individuals with periodontal health, patients with severe periodontitis are at a elevated risk of developing diabetes [6].
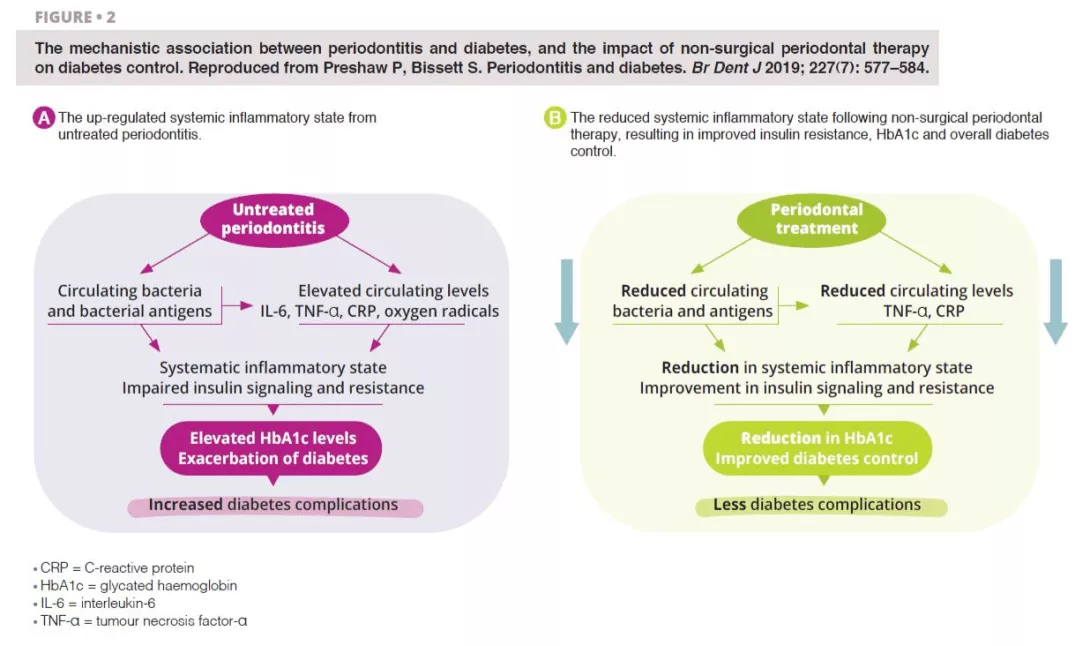
Untreated severe periodontitis is associated with an increase in the circulating levels of bacteria and bacterial antigens, pro-inflammatory mediators and cytokines, and increased levels of interleukin 6, tumour necrosis factor alpha, C-reactive protein, and oxygen free radicals. This combined effect fosters the conditions for systematic inflammation, impairing insulin signalling and resistance [6]. Clinically, this is recognised through increased HbA1C and the progression of diabetes, with greater risk of diabetic complications. Periodontal treatment reduces the oral bacterial load, and therefore lowers the circulating levels of inflammatory mediators, thereby reducing the degree of the systematic inflammatory state (figure 2). Hence, the dental management of periodontitis can lead to a clinically-relevant improvement in glycemic control, where patients with diabetes experience HbA1c reductions of 0.3–0.4% up to four months after treatment.
Atherosclerosis describes the accumulation of fats, cholesterol, and blood cells that form hardened plaque deposits within the artery walls, occluding blood flow through the vessels, increasing the risk of cardiovascular complications.
Oral bacteria are contributory infectious agents in the pathogenesis of atherosclerosis, through the invasion of cardiovascular host cells, namely endothelial cells [7].
Chronic periodontal disease can lead to endothelial dysfunction, through an elevated systematic inflammatory state, which can be shown through increased levels of IL-6, fibrinogens, and periodontopathic bacterial products, such as outer membrane vesicles and gingipains [8]. Much of the atherosclerotic pathology appears to be attributable to Porphyromonas gingivalis. However, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Tannerella forsythia and Fusobacterium nucleatum have each been studied in relation to this association. The primary microbial implications are endothelial dysfunction and the promotion of atherosclerosis in cardiovascular cells. P. gingivalis has the ability to attach to endothelial target cells, and external factors mediate its cellular entry, where it induces pro-coagulant effects. The results of a parallel-group, single-blind, randomised, controlled trial found that although intensive periodontal therapy led to systemic inflammation and endothelial dysfunction in the immediate term, 6 months after treatment, clinical and biochemical improvements in endothelial function were noted [9]. This study added to the theory that periodontal control may modulate atherosclerotic cardiovascular processes.
Rheumatoid arthritis is a chronic, autoimmune inflammatory condition affecting the synovial fluid of joints in a symmetrical pattern, and if untreated, other organs. Porphyromonas gingivalis is implicated in the pathophysiology of rheumatoid arthritis, where the bacteria produce enzymes with the capacity of citrullinating proteins, increasing the probability of reductions in the host immune tolerance and promoting the release of autoantibodies characteristic to the condition [10].
82% Cross-sectional data from the United States showed an 82% increase in rheumatoid arthritis associated with periodontitis, identified through gain in periodontal attachment loss [12].
Several studies have shown that periodontitis caused by dysbiotic oral biofilms, can trigger rheumatoid arthritis with systemic inflammation and increased bone erosion. A bidirectional relationship has been postulated between the inflammatory conditions, but further evidence is needed to verify this [11]. Clinicians involved in the rheumatological care of arthritis patients should be aware of the role of periodontitis as a factor modulating the efficacy of biologic disease- modifying anti-rheumatic therapies, since the maintenance of systemic inflammation could affect treatment response.
Non-surgical periodontal therapy appears to improve the biochemical expression of rheumatoid arthritis, but its role in improving clinical outcomes remains to be fully understood.
In patients with dysbiotic oral biofilms, where the proportions of periodontopathic bacteria capable of citrullinating proteins are higher than in health, preventive and curative treatment to stabilise the oral microbiome and periodontal inflammation would be prudent to include as a core aspect of the rheumatological care plan.
Scientific advances in the understanding of the oral microbiome demonstrate its contribution to both oral and general health and well-being. The ecological plaque hypothesis is the currently accepted theory desiring microbiological changes in the mouth, where shifts in the ecology of the oral microbiome result in disharmony, which foster key harmful pathogens to increase in number [13]. The dissemination of oral bacteria to systemic bodily sites is substantially reduced by enhancing control of the oral microbial load. Daily mechanical removal of plaque, through a systematic and comprehensive toothbrushing and interdental cleaning technique, reduces the volume of this load, and prevents the colonisation of pathogenic species. Good plaque control also prevents the risk of developing periodontal diseases, characterised by micro-ulceration of the gingival architecture, thereby producing channels for the leakage of bacteria and inflammatory mediators.
Supplemented with professional interventions by dental practitioners (commonly oral hygiene instruction, risk factor control, and mechanical plaque removal), periodontal disease processes can be stabilised and if mild, reversed.
Where the microbial balance has been disturbed in disease, the symbiotic equilibrium of the oral microbiome can be re-established and stabilised through relatively simple personal and professional interventions.
Research exploring the association between changes in the oral microbiome and systemic chronic conditions continues to expand.There are multiple plausible reasons to justify the bidirectionality of these putative connections. Dysbiosis in the oral microbiome, the primary driver for local and general disease onset and progression is mediated by highly modifiable risk factors, reinforcing the value of prevention, and the need for health systems to re-orient their mode of care delivery to accommodate the delivery of preventive oral health care.
1. Kilian M, Chapple IL, Hannig M, et al.. The oral microbiome - an update for oral healthcare professionals. Br Dent J 2016; 221: 657–66.
2. Patel J, Sampson V. The role of oral bacteria in COVID-19. Lancet Microbe 2020; 1: e105.
3. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–22.
4. Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012; 55: 21–31.
5. Teeuw WJ, Kosho MX, Poland DC, Gerdes VE, Loos BG. Periodontitis as a possible early sign of diabetes mellitus. BMJ Open Diabetes Res Care 2017; 5: e000326.
6. Preshaw P, Bissett S. Periodontitis and diabetes. Br Dent J 2019; 227: 577–84.
7. Tonetti MS, Van Dyke TE; working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013; 84(Suppl 4): S24–S29.
8. Reyes L, Herrera D, Kozarov E, Roldán S, Progulske-Fox A. Periodontal bacterial invasion and infection: contribution to atherosclerotic pathology. J Clin Periodontol 2013; 40 (Suppl 14): S30-S50.
9. Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007; 356: 911–20.
10. Quirke AM, Lugli EB, Wegner N, et al. Heightened immune response to autocitrullinated Porphyromonas gingivalis peptidylarginine deiminase: a potential mechanism for breaching immunologic tolerance in rheumatoid arthritis. Ann Rheum Dis 2014; 73: 263–9.
11. González-Febles J, Sanz M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol 2000 2021; 87: 181–203.
12. de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol 2008; 35: 70–6.
13. Marsh PD. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 1994; 8: 263–71.
The male urethra, which has been little described until now, is also thought to harbor microbiota. Vaginal intercourse could reshape its composition, making it a reservoir for the bacteria responsible for vaginosis in women.
Little is known about male urethral microbiota, since sampling is painful and often reserved for men with sexually transmitted infections (STIs). However, there is increasing evidence to suggest that microorganisms colonize this mucosa, even in healthy individuals. An American study including 110 men with no urethral symptoms, infections or inflammation has finally uncovered them.
Most of the individuals were heterosexual. A swab was inserted 2 cm into each man’s urethra to analyze the microbiota (shotgun sequencing). In total, 117 different species of bacteria were detected. Most of the samples contained lactic acid bacteria (e.g., Streptococcus mitis) and corynebacteria, which could represent the “core” microbiota responsible for the health of the urethra. But that’s not all. Scientists have also identified bacteria in some men that are associated with bacterial vaginosis in women, in particular Gardnerella vaginalis. As a consequence, the male genital tract could be colonized by bacteria that are potentially pathogenic for women, even though on the whole its microbiota differs greatly from that of the vagina.
10% of the microbiota present in the male urethra is influenced by sexual intercourse, in particular vaginal penetration.
Two types of urethral microbiota (or urethrotypes, UT) were identified: type 1 microbiota (UT1) which is less abundant and diversified, mainly dominated by S. mitis, and type 2 microbiota (UT2) which is more abundant and diversified, dominated by G. vaginalis and composed of nine bacteria associated with vaginal disorders (bacterial vaginosis, vaginitis, etc.) and capable of forming biofilms with G. vaginalis. Given the bacteria’s degree of affinity for oxygen, researchers believe that these two microbiomes are located in different niches: UT1 is located close to the urinary meatus and UT2 slightly deeper.
In addition, UT2 is associated with vaginal intercourse and certain bacteria related to bacterial vaginosis are still detectable 60 days after sexual intercourse and, to a lesser extent, after a year or even over a lifetime. Vaginal intercourse in the last two months alone explains 4.26% of the variance in the composition of male urethral microbiota. And sexual practices as a whole (oral, vaginal, anal) account for around 10% of this variance.
Finally, no other variable, whether anal or oral intercourse, age, ethnicity or history of STI, was associated with either urethrotype.
The male urogenital microbiota could therefore be linked to sexual behavior and the male urethra could, in some men, harbor a wide range of agents that are potentially pathogenic for the female vaginal flora. Could they represent a reservoir for sexually transmitted microorganisms and risk contaminating women during unprotected sex? If so, shouldn't bacterial vaginosis be considered an STI? Currently it is not considered as such but this hypothesis (which is not new) will be explored in further research involving couples this time.

"Yes. Experts who contribute to the Embedded/Chronic UTIs support group on FB say the same about what used to be known as ‘Interstitial’ Cystitis." - Hazel Bowden (From Biocodex Microbiota Institute on X)
 Idiopathic urethritis in men: new infectious etiologies?
Idiopathic urethritis in men: new infectious etiologies?
The microbiota of the male urethra has been little studied and yet it has the potential to disrupt the vaginal bacterial ecosystem. How? Read on to find out more.
The male urethra, i.e., the canal that runs through the penis and carries urine and sperm, is, like many other parts of the body, home to a whole microscopic world. This microbiota is generally only talked about when it is invaded by pathogenic bacteria that cause painful inflammation, such as the “clap” caused by gonococcal infection. Poorly understood and little studied (understandably, few men volunteer to be swabbed...), the microbiota of the male urethra has just revealed some of its secrets, thanks to the courage of 110 healthy Americans who agreed to have a swab taken.
The urethra, i.e., the small canal that connects the bladder to the tip of the penis, measures around 15 cm in men, compared with 3.5 cm in women.
The samples show that most of these men harbor a relatively simple community of (sidenote: Microorganisms Living organisms that are too small to be seen with the naked eye. They include bacteria, viruses, fungi, archaea and protozoa, and are commonly referred to as “microbes”. What is microbiology? Microbiology Society. ) , including oxygen-loving bacteria which probably live at the tip of the penis close to the open air. Their relatively constant presence suggests that they form a sort of core community, ensuring the health of the penile urethra.
However, some men have a more complex set of microorganisms, including bacteria known to disrupt women’s vaginal bacterial ecosystem and cause all sorts of disorders (vaginosis, etc.).
These bacteria could live a little deeper in the urethra of the penis, far from any source of oxygen. More importantly, only men who reported having had vaginal sex are carriers of these pathogenic bacteria. It's only a short step from there to imagining that they brought them back from a “close encounter” with a vagina...
Based on the findings of the researchers who examined the reported sexual practices of the 110 participants, sexual behavior (i.e., vaginal, oral or anal intercourse and combinations thereof) over the last two months explained around 10% of the total variation in the composition of the penile urethra microbiota, of which 4.26% can be explained by vaginal intercourse alone. Men in good health appear to be able to be colonized for extended periods by bacteria that they could pass on to their subsequent female partners, with these women running the risk of getting bacterial vaginosis. This is a public health issue that the team is continuing to investigate, with plans to study couples to find out more about potential transmissions.
 Urethral microbiota: a better understanding of male urinary tract infections
Urethral microbiota: a better understanding of male urinary tract infections
 Are men involved in bacterial vaginosis?
Are men involved in bacterial vaginosis?
A team of Irish researchers has recently discovered that the gut microbiota of people suffering from social phobia has specific characteristics that differ from those of healthy individuals 1. A first!
The gut microbiota Psychiatric disorders
We know that the gut and the brain are in constant dialog. Studies suggest that the “ microbiota-gut-brain axis ” plays an important role in anxiety, stress, major depressive disorder and autism.
What about the microbiota of people with social phobia? Does it also display characteristics suggesting it plays a role in the disease via communication with the brain?
Until now, a lack of data has made it difficult to answer this question. However, a study by a team of Irish researchers from University College Cork (UCC) suggests that this may indeed be the case.
The scientists enrolled 31 people diagnosed with social phobia, together with 18 controls not suffering from the condition. They collected the participants’ stools to analyze and compare the composition of their microbiota. What did the analyses show?
13% The percentage of the population in Europe and the United States affected by social phobia.
(sidenote:
Fehm L, Pelissolo A, et al. Size and burden of social phobia in Europe. Eur Neuropsychopharmacol. 2005;15:453–62.
Kessler RC, Petukhova M, et al. Twelvemonth and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84.
)
Compared to the control group, the microbiota of the “social phobia” group contained more Anaeromassilibacillus. Several studies have shown this bacterial genus to be implicated in autism and depression, two disorders that share physiological processes with social phobia.
Their microbiota also contained more Gordonibacter, a bacterial genus that can produce the metabolite urolithin from the breakdown of polyphenols, which studies show has an impact on mental health.
The social phobia group also had lower levels of Parasutterella excrementihominis. Several studies have shown this bacterium to be less abundant in people suffering from autism spectrum disorders, but also in those with a high (sidenote: Indice de masse corporelle Rapport du poids en kg sur le carré de la taille en m2 ) or sugar intake, which is common in people suffering from phobias, including those in the study.
Another notable difference was that their microbiota showed a significant enrichment in a metabolic pathway for aspartate degradation via a specific protein. According to the authors’ analysis, this protein is similar to another involved in the metabolism of tryptophan, one of the messengers involved in the functioning of the gut-brain axis.
Social phobia, also known as social anxiety disorder, is a persistent and intense fear of:
The fear is intense and pervasive and is accompanied by particularly disturbing physical symptoms: trembling, palpitations, excessive sweating, nausea, and at times panic attacks.
Social phobics may be unable to do things as simple as going to a restaurant, asking for directions, taking an exam, or attending a meeting. They are also at greater risk of alcoholism and depression.
This illness differs from stage fright or shyness in the intense psychological suffering it causes.
Treatment relies mainly on medication (antidepressants, beta-blockers) and cognitive behavioral therapies (CBT). 2
According to the researchers, this small study is highly important and will lay the foundations for larger-scale studies to confirm the involvement of the gut-brain axis and specific bacteria in social anxiety disorder.
The ultimate aim is to identify biomarkers and develop new treatments for social phobia, a common and particularly disabling disorder, for which treatments are not yet very effective.
News, accrediting training, infographics, expert’s video, thematic folder… Let’s deep dive to the Biocodex Microbiota Institute’s materials dedicated to microbiota gut-brain axis. Tools and contents adapted to your practice to improve your knowledge and be(come) easily a gut-brain axis expert!

"I will learn more about this field. " -@LiLNguyenVu (From Biocodex Microbiota Institute on X)
 Everything you need to know about Dysbiosis
Everything you need to know about Dysbiosis
 Everything you need to know about Irritable Bowel Syndrome (IBS)
Everything you need to know about Irritable Bowel Syndrome (IBS)


