Vaginal Microbiota # 16
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland



By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland

Each year, nearly 90 million cases of gonorrhea are reported worldwide. In women, infection of the lower genital tract by Neisseria gonorrhoeae has highly variable consequences, from no symptoms at all to cervicitis. Although the factors behind this variability are not known, the cervico-vaginal microbiota may be involved. In fact, a team has recently shown that the cervico- vaginal microbiota predicts the clinical presentation of gonorrhea in women.
These are the results of a pilot study in the US on 19 patients infected with N. gonorrhoeae, 10 of whom were symptomatic and 9 asymptomatic. Most of these patients were African American, a population whose microbiota is more frequently low in lactobacilli than that of Caucasian women. Neisseria spp. accounted for only 0.24% of the bacteria present in all 19 patients, whether symptomatic or asymptomatic. Half of the patients in each group also had co-infections with Chlamydia trachomatis and/or Trichomonas vaginalis.
The cervico-vaginal microbiota of the asymptomatic patients with no co-infection more frequently contained microbial communities dominated by lactobacilli (92.2% of bacteria on average) than that of the symptomatic patients with no co-infection (21.6%).
In contrast, the symptomatic women had microbial communities characterized by more diverse and heterogenous bacterial taxa. They were composed of a mixture of anaerobic bacteria associated with bacterial vaginosis (BV): Prevotella, Sneathia, Mycoplasma hominis and Bacterial Vaginosis- Associated Bacterium-1 (BVAB1) / Candidatus Lachnocurva vaginae.
These results are merely those of a pilot study based on a small sample. This is a crucial first step, but further studies are needed to evaluate the potentially protective effect against N. gonorrhoeae infection of a Lactobacillus-dominated vaginal microbiota.
By Pr. Satu Pekkala
Academy of Finland Research Fellow, Faculty of Sport and Health Sciences, University of Jyväskylä, Finland
Immune checkpoint inhibitors (ICIs) have significantly improved the treatment of advanced melanoma. However, not all patients respond to the treatment, which has been thought to be linked to the gut microbiota. Lee et al. performed metagenome shotgun sequencing of fecal samples from five European ICI-naïve cohorts, with a total of 165 advanced cutaneous melanoma patients. Due to clinical and mutational differences between the cohorts, they were analyzed separately and not pooled. The authors found a significant difference in the gut microbiota composition between responders and non-responders in the PRIMM-UK cohort, but not in the PRIMM-Netherland (NL) cohort. Further, by analyzing publicly available databases (n = 147 metagenomic samples), it became evident that there was limited reproducibility of response predictions across cohorts. No single bacterium was an entirely constant biomarker of response to ICIs across all datasets. However, a panel of microbial species, including Bifidobacterium pseudocatenulatum, Roseburia spp. and Akkermansia muciniphila, was identified in the study associated with responders. Regarding the functional genes of the microbiota, e.g., DNA adenine methylases were increased in the responders. In conclusion, though a potential panel of microbial biomarkers showing the responsiveness to ICI treatment was identified, future studies in larger cohorts are needed. In addition, several clinical factors should be considered as confounding when evaluating the biomarkers that may be useful in diagnostics
Many patients with non-small-cell lung cancer (NSCLC) do not respond to the treatment with immune checkpoint inhibitors (ICIs), such as anti-PD-1. Recent evidence shows that certain members of the gut microbiota, especially Akkermansia muciniphila, can influence the efficacy of ICIs in patients with NSCLC. In addition, the treatment resistance has been associated with lower inflammatory tumor microenvironment. Derosa et al. prospective, multicentric study included 338 patients with advanced NSCLC treated with ICIs to determine whether the gut microbiota metagenome profiles could explain the treatment response. They show that higher Akkermansia abundance in the baseline feces was associated with higher response rate to the ICI treatment, associated with clinical benefit (increase in survival). In addition, the presence of Akkermansia associated with other potentially prognosis- relevant shifts in the gut microbiota. Several differentially expressed tumor genes were linked to the response to PD-1 blockade suggesting that Akkermansia could promote migration of T helper cells to the tumor microenvironment. To ultimately show that Akkermansia could rescue the ICI resistance, the authors inoculated two different strains of A. muciniphila to mice that were previously transplanted with feces of a patient with resistance to PD-1 blockade. Compared to the control mice, the two strains rescued the treatment response. By far, this study is the largest metagenomics prospective analysis that has validated Akkermansia as a potential prognostic factor for ICI-treated NSCLC patients and showed the mechanistic potential of Akkermansia.
The only validated indication for FMT is recurrent Clostridioides difficile infection, However, the involvement of gut microbiota in numerous other diseases (Parkinson disease for example) suggests that indications for FMT could soon be expanded.Gut microbiota can also modify numerous depression-associated processes, such as hypothalamic- pituitary-adrenal gland axis. So far, there are no published trials using FMT to treat patients with bipolar conditions. The longitudinal study by Parker et al. presents the case of a 28-year-old male with bipolar disorder. At the age of 10, he developed depressive episodes. Symptoms included a severely depressed mood, suicidal thoughts, anergia, impaired concentration, psychomotor retardation, and insomnia. The symptoms were commonly associated with irritability and anxiety. At the age of 15, he developed his first hypomanic episode. For years, he was successfully treated with drugs, but then mood problems occurred again. He voluntarily started taking probiotics (strains of Lactobacillus and Saccharomyces. After the probiotics, he self-reported a huge relief of symptoms. Encouraged by the improvements, the patient read on microbiome research and elected to trial FMT. This procedure was performed via colonoscopy by a gastroenterologist. After the FMT, he charted his mood states for 470 consecutive days. He self-reported that mood episodes decreased in frequency and severity over the months. He was also able to reduce the drug treatment significantly. Twelve months after FMT, he stated having distinct highs, virtually no bipolar symptoms, and attention deficit hyperactivity disorder symptoms had improved. Though this is only a case study, FMT was able to reduce the bipolar symptoms, thus warranting the need for FMT studies in larger bipolar cohorts.
Commented article - Children's section
By Pr. Emmanuel Mas
Gastroenterology and Nutrition Department, Children’s Hospital, Toulouse, France

The early life gut microbiome plays a critical role in host development and influences brain functioning. This study investigated the association between gut microbiome succession from the first week of life and head circumference growth (HCG). Faecal samples were collected weekly from a preterm infant cohort in order to evaluate gut microbiome composition, in conjunction with clinical data and head circumference measurements. Preterm infants with suboptimal HCG trajectories had a depletion in the abundance/prevalence of Bacteroidota and Lachnospiraceae, which was unrelated to morbidity and caloric restriction. This article provides evidence that their integration into the gut microbiome needs to occur early for optimal neurodevelopment.
Neurodevelopment disorders are frequent in young children, affecting up to 8.4% of individuals under 5 years of age worldwide. Head circumference growth (HCG) is a marker correlated with early neurodevelopment.
It is important to seek environmental factors which could be modified to reduce neurodevelopment disorders. Interventional nutrition studies have failed to show significant results on neurodevelopment (e.g.: the benefit of breast feeding). The authors investigated the gut microbiome (GM) because its acquisition during the first months of life and the use of antibiotics during the first year are associated with various pathologies including neurodevelopment disorders later in childhood, in particular attention deficit hyperactivity disorder and autism spectrum disorder. The study objective was to determine whether there is a relationship between the characteristics of the early GM and a suboptimal trajectory of the HCG (SHCGT).
Infants of < 37 weeks postmenstrual age (Chicago neonatal care unit) were included between January 2010 and December 2018. HCG trajectory was the difference between head circumference z-scores measured at birth and at 36 weeks postmenstrual age (PMA); 0.5 z-score interval losses were used to define the groups having an appropriate HCG trajectory (AHCGT) or a suboptimal trajectory (mildly, moderately and severely impaired, SHCGT).
The β diversity of the GM differed significantly between SHCGT and AHCGT infants, as did the change in abundance of the taxa in the stool samples at 30 PMA. The loss in HCG z- score > 0.5 occurred between 31 and 36 weeks PMA in the SHCGT groups. This suggests that an «immature» GM precedes the SHCGT.
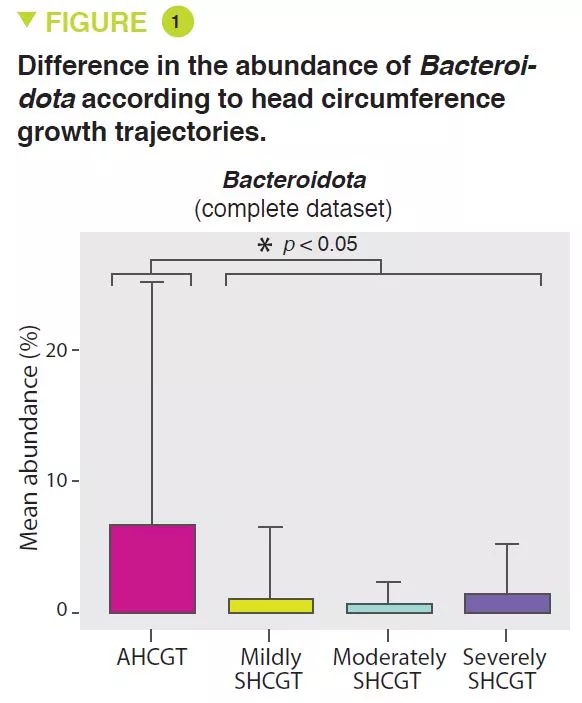
SHCGT infants had a significantly decreased abundance of Bacteroidota (p = 0.0009) (Figure 1) and Lachnospiraceae (p = 0.009), between 31 and 36 weeks PMA, which may result in a reduced carbohydrate utilisation capacity of these taxa. The prevalence of the Ruminococcaceae family (p = 0.007) was attributed to the species Faecalibacterium prausnitzii (p = 0.004), 48% in the AHCGT vs 8% in the SHCGT infants. It is to be noted that there was an increase in Firmicutes in the SHCGT groups from 24 to 30 weeks PMA (p = 0.009) but without any difference in the sub-taxa.
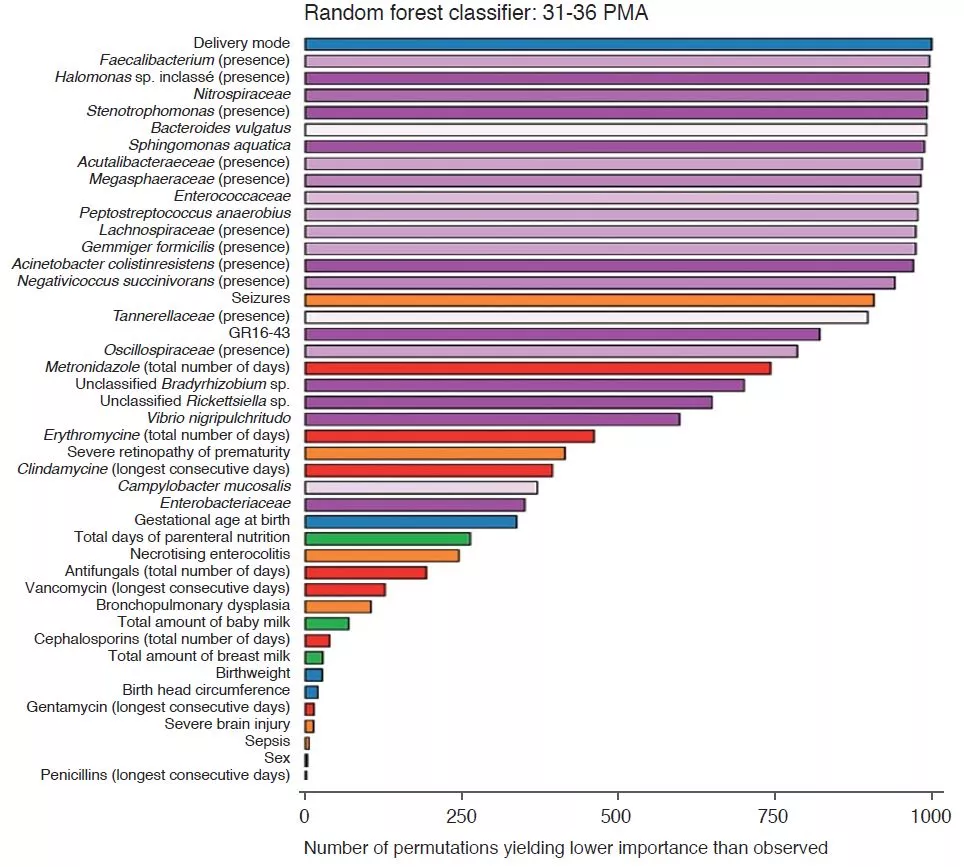
Analysis of the clinical parameters showed that changes in HCG were not due to caloric restrictions. There were more morbidities in the children of the SHCGT groups than in those with AHCGT: necrotising enterocolitis (p = 0.0006), severe neurological injury (p = 0.01), sepsis (p = 0.03). However, the analytical methods used, such as the random decision forest with permutations, showed that the most important factors associated with HCG trajectories were the characteristics of the GM rather than the comorbidities, whether from 24 to 30 PMA or from 31 to 36 PMA (Figure 2). In infants free from severe morbidities, the differences in Bacteroidota and Lachnospiraceae were still present but the abundance of Actinobacteriota was significantly greater in the AHCGT and in the mildly SHCGT than in the moderately and severely SHCGT groups.
Delivery mode had more effect on HCG trajectories than factors influencing GM such as enteral nutrition and antibiotic treatments. This is linked to the transmission of the GM at delivery because the abundance of Bacteroidota was greater in vaginally delivered infants than in those delivered by caesarean section. Moreover, among the vaginally delivered infants, those with a SHCGT had a fall in the abundance of the taxa described above as related to HCG trajectories, compared with the AHCGT. Moreover, the birth term is an important factor because all the vaginally delivered SHCGT infants were born < 27 weeks postmenstrual age, whereas only 17% of the vaginally delivered AHCGT infants were born < 27 weeks postmenstrual age.
It is suggested therefore that SHCGT starts with a reduction in the abundance of Bacteroidota and Lachnospiraceae, and then is exacerbated with the reduction in Actinobacteriota.
Vaginal birth enables the vertical transmission of Bacteroidota.
One must however be vigilant concerning infants born before 27 weeks PMA because even those delivered vaginally appear to be at a higher risk of SHCGT.
Studies designed to optimise GM from the first days of life in very premature infants may confirm and refine these results.
The gut microbiome is an important factor influencing head circumference growth trajectory. The very early acquisition of certain bacteria (Bacteroidota and Lachnospiraceae), enhanced by a vaginal delivery, may reduce neurodevelopment disorders.
COMMENTED ARTICLE - ADULTS’ SECTION
By Pr. Harry Sokol
Gastroenterology and Nutrition Department, Saint-Antoine Hospital, Paris, France

The fungal microbiota (mycobiota) is an integral part of the complex microbial community colonising the mammalian gastro-intestinal tract and has an important role in immune regulation. Although changes in the mycobiota have been linked to several diseases, including inflammatory bowel disease (IBD), it is currently unknown whether fungal species identified by sequencing represent living organisms and whether specific fungi have effects on the development of IBD. The authors developed a translational platform for the function al analysis of the mycobiome. By combining high-resolution mycobiota sequencing, fungal culturomics and genomics, a CRISPR-Cas9-based fungal strain editing system, in vitro functional immunoreactivity assays and in vivo models, this platform enables the examination of host-fungal crosstalk in the human gut. We discovered a rich genetic diversity of opportunistic Candida albicans strains that dominate the colonic mucosa of patients with IBD. Among these isolates, strains with high immune-cell-damaging capacity (HD strains) reflect the disease features of patients with ulcerative colitis and aggravate intestinal in flammation in vivo through IL-1β-dependent mechanisms. The inflammatory and antifungal responses of interleukin-17A-producing T helper cells (Th17 cells) induced by the HD strains in the gut were dependent on candidalysin, the peptide toxin secreted by C. albicans during the transition from a benign commensal to a pathobiont state. These findings reveal the strain-specific nature of host-fungal interactions in the human gut and highlight new diagnostic and therapeutic targets for IBD.
Studies based on deep sequencing of the gut mycobiota in several cohorts of patients supply coherent evidence that «fungal dysbiosis» is a characteristic of chronic inflammatory bowel disease (IBD) [2], the most widespread forms of which are Crohn’s disease (CD) and ulcerative colitis (UC), which affect millions of individuals worldwide. Anti-Saccharomyces cerevisiae antibodies (ASCA), directed against the mannans presented by the cell wall of fungi, define sub-types of IBD, because their presence in the serum is associated with CD but not with UC, which establishes an additional link between fungi and IBD. Candida is the most widespread fungal genus, and its presence is systematically increased in several cohorts of patients with IBD analysed by sequencing of the faecal microbiota [2].
In particular, C. albicans in the gut induces a set of antifungal antibodies and acts as an immunogen for ASCA. Candida species associated with the gut mucosa are detected by the macrophages present in the gut and have been shown experimentally to have the potential to induce protective immunity or to trigger inflammation, depending on the context [3]. Despite this evidence, it is currently unknown whether the fungi detected by sequencing technologies in the human gut have an essential role in the orientation of mucosal immunity or in the evolution of inflammatory disease in each individual patient. A lack of correlation has been repeatedly observed between changes in the composition of the mycobiota and the seriousness of the disease in IBD patient cohorts, despite a constant increase in Candida species. The authors have therefore emitted the hypothesis that the functional diversity of Candida strains determines the host-fungal relationship in human gut mucosa with an effect on intestinal inflammation.
In agreement with numerous other studies, the authors first observed that the mycobiota of patients with UC were rich in Candida albicans and, in contrast, poor in Saccharomyces. In the situation of impaired immune response induced by corticosteroid therapy, C. albicans exacerbates the severity of colitis in mice. The authors then isolated several strains of C. albicans from the mycobiota of healthy subjects and from patients with UC and observed a wide heterogeneity in terms of pro-inflammatory capacity. In particular, the capacity of causing cell damage to the macrophages, which are a key defence against fungi, varies from one strain to another. The strains able to inflict cell damage to the macrophages have a greater tendency to filament and have pro-inflammatory effects in vivo by inducing a Th17 response (Figure 1). The authors then showed that a large part of the pro-inflammatory effects were mediated by the secretion of a toxin, candidalysin, and the induction of IL-1β production. Subsequent analyses revealed a strong correlation between the pro-inflammatory capacity of strains isolated from patients with UC and the inflammatory activity of the disease. On the other hand, there was no correlation between the magnitude of intestinal inflammation and the overall abundance of Candida albicans in the patients. These results explain why the composition of the mycobiota is poorly correlated to the characteristics of human pathologies and suggest that the functional capacities (pro-inflammatory in this case) may provide a better explanation of the role of the mycobiota in these pathologies.
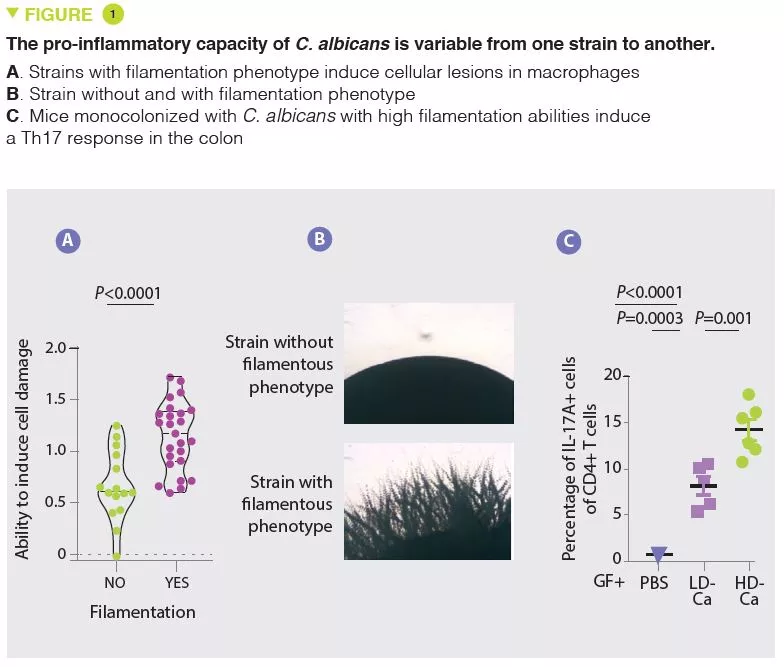
This study shows that, in addition to analyses of the composition of the mycobiota, in particular by sequencing, a functional analysis is necessary to understand its contribution to the disease and in particular in IBD. If pro-inflammatory strains of C. albicans, candidalysin and IL-1β are confirmed to have a role in UC, we can imagine a therapeutic option which targets one of these elements, especially as several molecules which antagonise the IL-1β pathway are already available.
This study suggests that candidalysin is a key factor in the pro-inflammatory effect of C. albicans in the gut and that some highly pro-inflammatory strains act via IL-1β-dependent mechanisms. Patients who are carriers of highly pro-inflammatory strains may represent a target population for a treatment antagonising IL-1 and/or C. albicans.
1. Li XV, Leonardi I, Putzel GG, et al. Immune regulation by fungal strain diversity in inflammatory bowel disease. Nature 2022 ; 603 : 672-8.
2. Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD. Gut 2017 ; 66 : 1039-48.
3. Doron I, Leonardi I, Li XV, et al. Human gut mycobiota tune immunity via CARD9-dependent induction of anti-fungal IgG antibodies. Cell 2021 ; 184 : 1017-1031.e14.
Overview
By Pr. Sian M. J. Hemmings
Department of Psychiatry, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, South Africa

Stress-related disorders, including posttraumatic stress disorder (PTSD), major depressive disorder (MDD) and anxiety disorders, are common psychiatric disorders with a dysfunctional response to stress a key pathogenic mechanism. These disorders are highly complex and debilitating, and are associated with increased mortality and morbidity. There is considerable evidence implicating the role of the gut microbiota in psychiatric disorders, including stress-related disorders. Delineating a specific gut microbial profile associated with the development of these psychiatric disorders may facilitate identification of reliable biomarkers of diseaseassociated risk and predict predisposition to develop such disorders. Moreover, the gut microbiota can easily be manipulated and could, therefore, offer a simple and sustainable treatment option to alleviate symptoms of stress-related disorders. This article reviews current literature on the microbiome-gut-brain axis, and how this bidirectional system of communication may play a role in the aetiology of PTSD, MDD and anxiety disorders.
Psychiatric disorders are chronic, debilitating disorders that significantly impair daily functioning, and are among the top ten leading causes of burden of disease worldwide [1]. Exposure to environmental stressors and trauma is associated with increased incidence of post-traumatic stress disorder (PTSD), major depressive disorder (MDD) and anxiety disorders [2, 3]. These stress-related disorders are associated with increased mortality, reduced life expectancy, are highly comorbid, and exhibit variable response to first line pharmacotherapy. There are no clinically actionable biomarkers for these disorders, further complicating their diagnosis and treatment. To facilitate the development of novel therapeutic strategies and potential interventions, it is imperative that we gain deeper insight into the biological mechanisms underlying these disorders.
“Microbiota” is the term referring to the trillions of microorganisms that live in and on us. The complete catalogue of these microbes and their genes constitutes the human microbiome. The gut microbiome, crucial in maintaining numerous aspects of our physiological functioning, is a dynamic system, the composition of which is affected by numerous factors including host genetics, age, diet and ethnicity [4-6]. The microbiome-gut-brain (MGB) axis is a complex, bidirectional system of communication between the gut microbiome, the gut, and the central nervous system (CNS), facilitated by direct and indirect communication pathways (Figure 1).
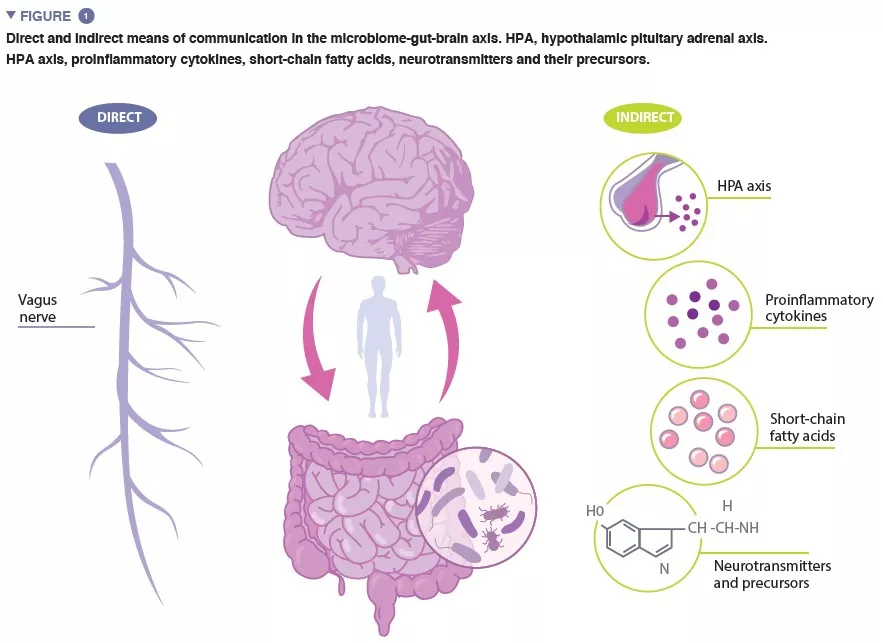
The vagus nerve, the major parasympathetic nerve of the autonomic nervous system, represents a direct link between the gut and the brain, with vagal afferents and efferents facilitating reciprocal interaction between the enteric nervous system and the brain. Indirect communication within the MGB axis takes on many forms. Microbiota produce several microbial-derived molecules, including neurotransmitters and metabolites, which act at multiple locations in the body. Many of these molecules, including serotonin (5-HT) have been found to regulate behaviour, brain function and health. As much as 95% of the 5-HT in the body is produced in the enterochromaffin cells lining the intestine, and 5-HT levels in the gut are influenced by microbial metabolites including indole, bile acids and short-chain fatty acids (SCFAs). 5-HT produced in the gut cannot bypass the blood-brain barrier (BBB), and therefore cannot affect 5-HT levels in the brain. However, animal studies provide evidence to suggest that levels of the 5-HT precursor, tryptophan, modulated by certain gut bacteria, are associated the regulation of 5-HT neurotransmission in the brain [7].
Alterations in SCFAs, a product of bacterial fermentation of host-undigestible polysaccharides, have been found to be associated with exposure to chronic stress and depressive-like behaviour in animal studies. SCFAs are involved in a number of regulatory functions, including modulation of gut activity and intestinal integrity, and activation of microglia (innate immune cells in the brain, which play an important role in regulating neuronal survival and responses). SCFAs are capable of crossing the BBB, and in doing so, may affect brain function.
It is well established that the gut microbiome plays an important role in the development of both the peripheral and central immune systems, and accumulating evidence suggests that increased inflammation is associated with stress-related disorders. An imbalance in gut microbial composition can compromise the integrity of the intestinal epithelium [8], increasing intestinal permeability and facilitating translocation of bacteria, or bacterial components, across the epithelial barrier into systemic circulation. This promotes low-grade inflammation, which stimulates increased expression of pro-inflammatory cytokines. Proinflammatory cytokines can stimulate the hypothalamic-pituitary adrenal (HPA) axis to secrete cortisol, which may further increase intestinal permeability. Indeed, evidence of gut and brain barrier dysfunction has been reported in stress-related disorders.
Systemic administration of lipopolysaccharides (LPS), a major component of the outer membrane of gram-negative bacteria, has been found to result in acute anxiety and increased depressive-like symptoms, as well as cognitive deficits, and LPS-induced increase in proinflammatory cytokine levels have been found to alter neuronal activity in limbic areas of the brain. LPS has also been found to induce increased cytokine production in the CNS, compromising the integrity of the BBB, resulting in a “leaky brain”.
Several preclinical investigations support the idea that the gut microbiome composition is associated with stress-related disorders. Investigations using germ-free (GF, microbiologically sterile) animals have played a crucial role in our understanding of the MGB axis. In their seminal investigation, Sudo and colleagues [9] observed an exaggerated stress response, evidenced by increased levels of corticosterone, in GF mice compared to controls, following acute restraint stress. This exaggerated HPA axis response to stress was normalised upon mono-colonisation of the GF mice with Bifidobacterium infantum. Studies have also shown that it is possible to transfer anxiety- like behavioural phenotypes between two mouse strains, by means of fecal microbiota transplant (FMT) [10]. Similarly, several studies have reported on the development of depressive- and anxiety-like behaviour, and altered neuroendocrine-immune pathways, in microbiota-depleted rodents following FMT from humans diagnosed with MDD, suggesting a causal role for gut microbiota in depressive-like behaviour [11-13]. Animal studies have also shown that exposure to stress can cause long-lasting alterations in the gut microbiome – two recent studies reported on decreased relative abundance of Akkermansia muciniphila in the gut microbiome of stressed animals over time, compared to control animals [14, 15]. A. muciniphila and the outer membrane coat of the bacteria (Amuc_1100) have been found to ameliorate depressive-like behaviour, and increase circulatory levels of 5-HT.
Comparatively few clinical studies have been conducted to determine the association between the gut microbiome and stress-related disorders. Thus far, the only published data on the gut microbiome in PTSD emanates from our research group [16], where a consortium of four bacterial genera was found to predict PTSD status with 66.4% accuracy. In addition, MDD diagnosis in the sample was found to be associated with increased relative abundance of the phylum Bacteroidetes. Other studies indicate that bacterial taxa associated with both depression and anxiety disorders are characterised by a higher relative abundance of taxa that induce a proinflammatory environment and a reduced abundance of SCFA-producing bacteria [17].
This field of research is, however, still in its infancy, currently limited by the lack of standardisation in gut microbiome analysis, from sample collection to the analytical pipeline. In many cases, factors that may confound results, including diet, medication use, ethnicity and host genetics, were not accounted for in the studies reviewed above. Moreover, most of the studies conducted have been cross-sectional in design, limiting our ability to disentangle cause from consequence, and very few have investigated potential mechanisms underlying the associations.
A. muciniphila is a gram-negative anaerobic bacterium, found primarily in the intestinal mucosa. It plays a role in maintenance of intestinal barrier integrity as well as in immune and metabolic regulation.
The gut microbiome is tractable and has the potential to be modulated, making the search for gut microbiome markers associated with stress-related disorders particularly attractive. Probiotics are defined as living microorganisms which, when administered in adequate amounts, confer a health benefit on the host; psychobiotics refer to probiotics which confer a benefit on mental health, cognition and behaviour. Recent publications have indicated moderate beneficial effects of psychobiotics in alleviating depressive and anxiety symptoms in both healthy and clinically-defined cohorts [18]. It is, however, important to remain cautious when interpreting results from current studies, as they are variable with regards to probiotic formulation and dosage, sample characteristics (clinical phenotype and severity of depression/anxiety), and follow-up time. In addition, the benefit of psychobiotics over, and interactions with, antidepressant medication has not yet been extensively investigated, although some intriguing results from preclinical studies suggest that certain probiotics, when administered in multi-strain format, possess antidepressant effects similar to, and sometimes with larger effect than, current first-line antidepressants [19]. Such psychobiotics, when used in conjunction with antidepressants, may have particular use in individuals with treatment-resistant depression.
Evidence to suggest that the gut microbiome is altered in stress-related disorders continues to grow, and while much work remains to be done in the field, delineating a specific gut microbial profile associated with the development of stress-related disorders may facilitate identification of reliable biomarkers of disease-associated risk and predict predisposition to develop these disorders. The gut microbiome can easily be manipulated and could, therefore, offer a simple and sustainable treatment option to alleviate symptoms of PTSD, MDD and anxiety disorders.
• 1. GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022; 9: 137-50.
• 2. van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 891-907.
• 3. Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 2010; 35: 169-91.
• 4. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 2010; 107: 14691-6.
• 5. Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G. Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014; 5: 3564.
• 6. Ayeni FA, Biagi E, Rampelli S, et al. Infant and Adult Gut Microbiome and Metabolome in Rural Bassa and Urban Settlers from Nigeria. Cell Rep 2018; 23: 3056-67.
• 7. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry 2013; 18: 666-73.
• 8. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010; 170: 1179-88.
• 9. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 2004; 558: 263-75.
• 10. Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141: 599-609.
• 11. Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016; 82: 109-18.
• 12. Liu S, Guo R, Liu F, Yuan Q, Yu Y, Ren F. Gut microbiota regulates depression-like behavior in rats through the neuroendocrine-immune-mitochondrial pathway. Neuropsychiatr Dis Treat 2020; 16: 859-69.
• 13. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016; 21: 786-96.
• 14. Hoke A, Chakraborty N, Gautam A, Hammamieh R, Jett M. Acute and delayed effects of stress eliciting post-traumatic stress-like disorder differentially alters fecal microbiota composition in a male mouse model. Front Cell Infect Microbiol 2022; 12: 810815.
• 15. Pascual Cuadrado D, Todorov H, Lerner R, et al. Long-term molecular differences between resilient and susceptible mice after a single traumatic exposure. Br J Pharmacol 2022; 179: 4161-80.
• 16. Malan-Muller S, Valles-Colomer M, Foxx CL, et al. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. Eur Neuropsychopharmacol 2022; 56: 24-38.
• 17. Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev 2021;83: 101943.
• 18. Alli SR, Gorbovskaya I, Liu JCW, Kolla NJ, Brown L, Müller DJ. The gut microbiome in depression and potential benefit of prebiotics, probiotics and synbiotics: a systematic review of clinical trials and observational studies. Int J Mol Sciences 2022; 23: 4494.
• 19. Ra Y, Eu P, Ev V, Mv O, Mv M, Gi K, et al. A Multi-strain potential probiotic formulation of GABA-producing Lactobacillus plantarum 90sk and Bifidobacterium adolescentis 150 with antidepressant effects. Probiotics and Antimicrobial Proteins 2020; 12: 973-9.
Mode of birth, diet, antibiotic exposure... Many factors are known to influence microbiota development and health in early childhood. A Danish study published in Microbiome1 has shown that siblings also play a major role.

From birth, the formation of an infant’s microbiota depends on its closest microbial sources, above all the mother, but also the rest of the family, particularly brothers and sisters. However, there have been few studies on the influence of siblings in this regard. To fill the gap, Danish researchers sequenced fecal (taken at 1 week, 1 month, 3 months, 1 year, 4 years, and 6 years) and pharyngeal samples (taken at 1 week, 1 month, and 3 months) from 686 children in the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) cohort. Data on younger and older siblings were collected or updated at each visit. In addition, fifteen covariates were identified, including birth weight, antibiotic use, diet, and the presence of pets. The researchers then assessed the relationship between the gut microbiota signature of having siblings and the presence of asthma, allergic rhinitis, and allergic sensitization at six years of age.
The researchers found siblings to be one of the most important factors in the development of a child’s gut and pharyngeal microbiota. Significant differences in composition, both in terms of diversity and quantity of bacterial genera, were found. The impact of siblings was particularly evident in the first year of life, and a smaller age difference with respect to the older sibling had a greater impact than the number of older siblings.
The pharyngeal microbiota of children with older siblings had a reduced alpha diversity at three months compared with that of only children. Moraxella and Neisseria were more abundant, whereas Staphylococcus was less abundant. No other factor, including even major factors such as breastfeeding or antibiotic use, had a greater impact on the composition of the pharyngeal microbiota than siblings.
As for the gut microbiota, children with older siblings showed a higher alpha diversity and a significant difference in beta diversity up to four years of age. At one year, siblings were the most important determinant of beta diversity after mode of birth. Children with older siblings had a significantly lower abundance of Escherichia/Shigella, other Enterobacteriaceae, and Veillonella, but a significantly higher abundance of Prevotella. Notably, the increased abundance of Prevotella was even more pronounced at four years and persisted at six years. Lastly, a gut microbiota with the sibling signature at one year of age was associated with a reduced risk of asthma at age six.
The researchers therefore believe that the development of an infant’s microbiota is significantly influenced by siblings, with consequences for the child’s health. They suggest that studies on the development of the microbiota in children should take into account the presence of siblings, especially older siblings.
A long and healthy life? It’s down to genetics, environment, and lifestyle. And something else as well? Scientific research is trying to unravel the complex interactions between these factors to help us understand aging and perhaps slow it down. With this in mind, a study on the gut microbiota of the world’s centenarians has brought some key answers to light. Focus on Sardinia.
The gut microbiota Diet
Our bodies change throughout our lives. This includes our gut microbiota, to the extent that the species that make it up can pinpoint our age. We know that the gut microbiota changes with age, losing diversity, and ultimately becoming unbalanced (dysbiosis), which may in turn contribute to the aging process. Conversely, maintaining a balanced microbiota may preserve the proper functioning of the body’s metabolism and immune system, but also contribute to the fight against inflammation and the decline of cognitive and bone health.
Since the gut microbiota is implicated in aging, can we learn something from the microbiota of those who live very long and healthy lives? To answer this question, Italian researchers1 traveled to the south of Sardinia, a region famous for its centenarians. As in other “blue zones” around the world, the researchers encountered men who had above-average longevity (and women with a longer life expectancy) while remaining in good physical shape.2 They hoped to discover the unique features of a gut microbiota associated with exceptional longevity, but also to evaluate the extent to which the microbiota can be inherited by descendants, and the impact of environmental factors such as diet.
The world’s “blue zones” are:4
The researchers analyzed the gut microbiota of a group of 46 dashing centenarians and nonagenarians, and compared it to a control group of 46 healthy younger adults (40-60 years old). They also compared the gut microbiota of seven centenarians with that of their children.
They found that the gut microbiota of the centenarians had a strong presence of bacteria from the Verrucomicrobiota phylum, particularly the species Akkermansia muciniphila. Among other things, this bacterium is known to contribute to immunity, metabolic health, and maintaining the integrity of the intestinal barrier. While the gut microbiota of the youngest participants was dominated by the Bacteroidetes phylum and in particular, the genus Bacteroides, that of the nonagenarians was associated with Actinobacteria, particularly Bifidobacteria. Moreover, the gut microbiota of the centenarians was closer to that of the youngest participants than that of the nonagenarians.
According to the researchers, the gut microbiota of the centenarians was a carefully balanced microbial community. It included species associated with intestinal health that oppose the action of pathogens and have an anti-inflammatory effect that benefits the entire body. In addition, its richness and complexity allow it to adapt to environmental disturbances.
The study showed the gut microbiota of the centenarians to be relatively similar to that of their children. The latter may therefore inherit characteristics of the microbiota, but also family lifestyle habits, particularly dietary habits, which predispose them to longevity. Moreover, a correlation was found between the species of the gut microbiota linked to longevity and adherence to a Mediterranean diet: further evidence of the benefits of this fiber- and anti-oxidant rich diet!
The way we age depends on the genes we inherit from our parents, but also on our environment and lifestyle: diet, smoking, physical activity, working conditions, etc. In particular, a healthy diet plays a major role in the balance of the gut microbiota and health, e.g. in the elderly, the Mediterranean diet reduces the risk of frailty and leads to better health.

"Interesting." - Bev Hansen (From My health, my microbiota)
Many factors play a role in the development of our microbiota and consequently in our health, e.g. birth by vaginal delivery or c-section, diet, antibiotic use. Although less well known, and less studied, siblings also play a major role. Danish researchers1 have recently found strong evidence to back this up.
The ENT microbiota The gut microbiota Asthma and microbiota Allergic rhinitis
From the moment we are born, the small world around us contributes to the unique composition of our microbiota. The microbes we are exposed to differ depending on whether we are born via c-section, are breastfed, grow up on a farm, have a dog... or have brothers and sisters! To assess the impact of siblings on the development of the microbiota, the researchers analyzed the composition of the gut flora (from 1 week to 6 years of age) and pharyngeal microbiota (from 1 week to 3 months of age) of nearly 700 children. The samples were regularly renewed, with nearly 4,500 samples sequenced in all. At each stage, the researchers took into account the child’s family situation, i.e. only child or presence of older/younger sibling(s). They also noted about 15 additional factors that may influence a child’s microbiota, from birth weight to household income. Lastly, they compared the data collected from the children with the presence of asthma, allergic rhinitis, and sensitization to various allergens at age six.
The researchers found that having siblings during early childhood was one of the most important determinants of the composition of the gut microbiota and airway microbiota. This effect was most pronounced in children in their first year of life with older siblings. Their gut microbiota was richer, more diverse, and more mature than that of only children. Furthermore, a large family is not necessary, since having one older sibling close in age mattered more than having a number of siblings. The consolation for only children: the difference between their microbiota and that of children with siblings subsides by age four.
What about the airway microbiota? During the first three months of life, it too was modified to a greater extent by the presence of a sibling than by other major factors, such as breastfeeding or antibiotic use. The microbiota of babies with siblings was less diversified than that of babies with no siblings. However, unlike the gut microbiota, lower bacterial diversity in the airways appears to be favorable to respiratory health.2
What does the study tell us? Having siblings in early childhood impacts microbiota development and health. On the other hand, siblings make it easier for children to be contaminated by microbes that cause colds and other illnesses. However, the researchers believe that early exposure to relatively harmless microbes can reduce the risk of allergic diseases.3
 The role of intestinal microbiota in respiratory infections
The role of intestinal microbiota in respiratory infections
Recent scientific publications have provided new data highlighting the key role of microbiota on women’s health. Biocodex Microbiota Institute is launching a set of expert interviews dedicated to microbiota, women and health. What do we already know about woman’s health and microbiota? What do we still have to discover?
First act: menopause and microbiota. Prof. Ina Schuppe Koistinen, microbiome researcher tells us everything!
Prof. Ina Schuppe Koistinen: Menopause is the time in a women’s life that marks the end of the reproductive period while for most many years of life remain. Menopause is defined retrospectively after a woman has ceased menstruation for 12 months.
Signs and symptoms of the menopause transition can vary widely among women. Many women experience irregular periods before they end. Other symptoms include hot flashes and chills, night sweats, sleep problems and mood changes. Low sex hormone levels increase signs of age, such as thinning hair, dry skin and loss of breast fullness. Genitourinary symptoms include vaginal atrophy and dryness.
The menopausal transition occurs over a time period of several years starting in the age between 40 to 50. During that time, also called perimenopause, the ovaries decrease their production of the female sex hormones estradiol and progesterone. After the final menstruation, ovarian follicles are depleted, and sex hormone levels stay low. However, it is crucial to remember that many women witness that life after 50 is their best time, with more freedom, no menses, no (sidenote: Premenstrual symptoms This refers to the symptoms women can experience in the weeks before their period (breast pain, mood swings, irritability, depression, fatigue…) Gudipally PR, Sharma GK. Premenstrual Syndrome. 2022 Jul 18. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan ) or pregnancies.
I. S.-K.: The vaginal microbiota in healthy women during the fertile years is dominated by (sidenote: Lactobacilli Rod-shaped bacteria whose main characteristic is the production of lactic acid, from where they get the name “lactic acid bacteria”. Lactobacilli are present in the oral, vaginal and gut microbiota of humans, but also in plants and animals. They are found in fermented foods, such as dairy products (e.g. certain cheeses and yoghurts), pickles, sauerkraut, etc. Lactobacilli are also found in probiotics, with certain species recognized for their beneficial properties. W. H. Holzapfel et B. J. Wood, The Genera of Lactic Acid Bacteria, 2, Springer-Verlag, 1st ed. 1995 (2012), 411 p. « The genus Lactobacillus par W. P. Hammes, R. F. Vogel Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004 Jun;70(6):3189-94. Smith TJ, Rigassio-Radler D, Denmark R, et al. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013 Jun;109(11):1999-2007. ) species that protect the vagina from overgrowth of other organisms.
The composition of the vaginal microbiota is influenced by estrogen that promotes the proliferation of vaginal epithelial cells and increases glycogen storage, the main nutrient for lactobacillus growth. With decreasing levels of estrogen during the menopausal transition, the diversity of the vaginal microbiota increases while lactobacilli decrease1,2.
Lactobacilli keep the vaginal pH low by production of lactic acid, produce antimicrobials such as H2O2 and bacteriocins. They also compete for nutrients, adhere tightly to the mucosal membrane and modulate the local (sidenote: Innate and adaptive immunity The human body protects itself using two kinds of defense mechanisms: innate immunity and adaptive immunity. Innate immunity is the first line of defense against disease agents and is an immediate response, while adaptive immunity is delayed but provides lasting protection Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Principles of innate and adaptive immunity. ) to protect the vagina from infections.
I. S.-K.: Women after menopause often suffer from urinary tract infections. Clinical data3 suggest an impact of estrogen on the pathogenesis of those infections. The low estrogen levels induce structural changes such as increased residual urine volume and changes in the vaginal microbiota as described above. Both are well documented risk factors for urinary tract infections. Local supplementation with estrogen can at least partly reverse these changes by the re-establishment of a lactobacilli-dominated vaginal microbiota and improved epithelial cell growth in the urogenital tract4.
I. S.-K.: There are very few research studies in the scientific literature that have described the changes of the gut microbiota after menopause transition. So far, data5 from samples including less than 200 women have been analyzed to study the effect of menopause on the gut microbiota and few changes have been detected. More high-quality studies with samples from thousands of women are needed to describe and understand the effect.
I. S.-K.: My advice is to take good care of your general health by lifestyle choices that promote a healthy microbiota. Taking good care of the gut microbiota will help you to keep a healthy vagina. Eat a varied diet rich in fiber and fermented foods, stay physically active and spend time in nature. Do not smoke and keep your alcohol consumption low, avoid the use of antibiotics if possible.
Regarding the vaginal microbiota, avoid washing your vulva with soap and antiseptics, never rinse your vagina with perfumes or detergents as it cleans itself by its continuous discharge. Wash with lukewarm water or with perfume- and soap-free cleaning products. Use a neutral vegetable oil to keep the sensitive mucosal membranes of your vulva lubricated. Practice safe sex and if you experience dryness in your vagina use lubricants when having sex and consider a local treatment with an estrogen-containing cream.
Do not hesitate to contact your gynecologist if you experience severe symptoms of menopause transition and your quality of life is affected.
I. S.-K.: It has been shown that women that passed menopause who underwent hormone replacement therapy had a similar lactobacillus-dominant composition of the vaginal microbiota to premenopausal women. With other words, hormone replacement therapy has a positive effect on the vaginal microbiota per se.
Prebiotics and probiotics could potentially contribute to a restoration of the vaginal microbiota to the state prior to menopause. This would require probiotics containing the right strains of Lactobacillus crispatus that are associated to good vaginal health.


Get free training on the early establishment of gut microbiota in this CME course, guided by Ericka Montijo. In this course, you will learn about the evolution and importance of the gut microbiota throughout our life.
In recent years, the interest in researching the complex interaction between the human gut microbiota and our health has not stopped. Moreover, healthcare professionals have begun wondering if the initial establishment of the gut microbiota plays any role in our adult life. How is the microbiota formed and developed in infancy? How important is it later in life? In this course, you will learn about which factors influence our microbiota even before we are born, and what the consequences of an immature microbiota are later in adult life. Moreover, do not miss out on the practical recommendations on how to enhance a healthy, mature microbiota in children!Unrestricted grant by Biocodex Microbiota Institute
Xpeer Medical Education is the first accredited medical education app in the market, with video microlearning engaging videos of just 5 minutes.
With a powerful algorithm to personalize the user experience and the contents as the most popular entertaining streaming platforms, it offers a brand new experience for the continuing education and professional development of the healthcare professionals.
Accredited by the European Union of Medical Specialists, it delivers high quality scientific medical education pieces. On Xpeer, you will find this curriculum on Microbiota and 500 hours of medical education in 2021 in your specialty, technologies and professional and personal skills.
The app Xpeer is accredited by the European Accreditation Council for Continuing Medical Education (EACCME) to provide official ECMEC credits recognized officially in 26 countries.
The credits for the users of the module will be 1 European CME credit (ECMEC®) for every hour (60 minutes of actual e-learning excluding introductions etc.) of use, provided that the users have completed a module and have passed the relevant assessment.






