Alcohol use disorders: in microbiota veritas?
Millions of people around the world drink alcohol on a regular basis, but not all develop alcohol use disorders. A study published in Translational Psychiatry1 suggests that gut microbiota composition may help explain inter-individual differences in drinking patterns.
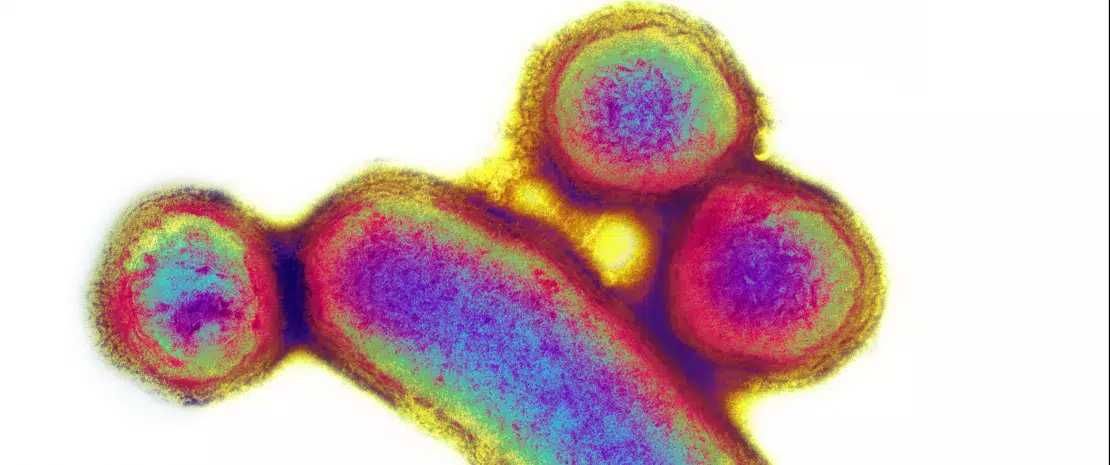
 Involvement of E. faecalis in alcoholic hepatitis
Involvement of E. faecalis in alcoholic hepatitis